Page 425 - Williams Hematology ( PDFDrive )
P. 425
400 Part V: Therapeutic Principles Chapter 25: Antithrombotic Therapy 401
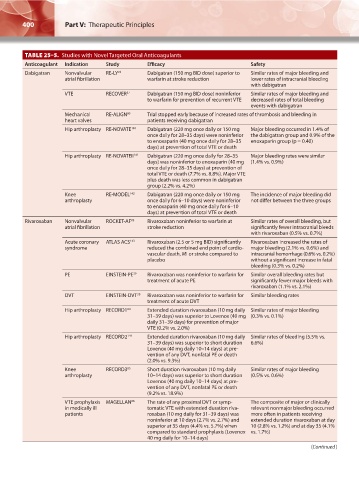
TABLE 25–5. Studies with Novel Targeted Oral Anticoagulants
Anticoagulant Indication Study Efficacy Safety
Dabigatran Nonvalvular RE-LY 78 Dabigatran (150 mg BID dose) superior to Similar rates of major bleeding and
atrial fibrillation warfarin at stroke reduction lower rates of intracranial bleeding
with dabigatran
VTE RECOVER 37 Dabigatran (150 mg BID dose) noninferior Similar rates of major bleeding and
to warfarin for prevention of recurrent VTE decreased rates of total bleeding
events with dabigatran
Mechanical RE-ALIGN 80 Trial stopped early because of increased rates of thrombosis and bleeding in
heart valves patients receiving dabigatran
Hip arthroplasty RE-NOVATE 140 Dabigatran (220 mg once daily or 150 mg Major bleeding occurred in 1.4% of
once daily for 28–35 days) were noninferior the dabigatran group and 0.9% of the
to enoxaparin (40 mg once daily for 28–35 enoxaparin group (p = 0.40)
days) at prevention of total VTE or death
Hip arthroplasty RE-NOVATEII 141 Dabigatran (220 mg once daily for 28–35 Major bleeding rates were similar
days) was noninferior to enoxaparin (40 mg (1.4% vs. 0.9%)
once daily for 28–35 days) at prevention of
total VTE or death (7.7% vs. 8.8%). Major VTE
plus death was less common in dabigatran
group (2.2% vs. 4.2%)
Knee RE-MODEL 142 Dabigatran (220 mg once daily or 150 mg The incidence of major bleeding did
arthroplasty once daily for 6–10 days) were noninferior not differ between the three groups
to enoxaparin (40 mg once daily for 6–10
days) at prevention of total VTE or death
Rivaroxaban Nonvalvular ROCKET-AF 83 Rivaroxaban noninferior to warfarin at Similar rates of overall bleeding, but
atrial fibrillation stroke reduction significantly fewer intracranial bleeds
with rivaroxaban (0.5% vs. 0.7%)
Acute coronary ATLAS ACS 143 Rivaroxaban (2.5 or 5 mg BID) significantly Rivaroxaban increased the rates of
syndrome reduced the combined end point of cardio- major bleeding (2.1% vs. 0.6%) and
vascular death, MI or stroke compared to intracranial hemorrhage (0.6% vs. 0.2%)
placebo without a significant increase in fatal
bleeding (0.3% vs. 0.2%)
PE EINSTEIN-PE 39 Rivaroxaban was noninferior to warfarin for Similar overall bleeding rates but
treatment of acute PE significantly fewer major bleeds with
rivaroxaban (1.1% vs. 2.1%)
DVT EINSTEIN-DVT 38 Rivaroxaban was noninferior to warfarin for Similar bleeding rates
treatment of acute DVT
Hip arthroplasty RECORD1 84 Extended duration rivaroxaban (10 mg daily Similar rates of major bleeding
31–39 days) was superior to Lovenox (40 mg (0.3% vs. 0.1%)
daily 31–39 days) for prevention of major
VTE (0.2% vs. 2.0%)
Hip arthroplasty RECORD2 144 Extended duration rivaroxaban (10 mg daily Similar rates of bleeding (5.5% vs.
31–39 days) was superior to short duration 6.6%)
Lovenox (40 mg daily 10–14 days) at pre-
vention of any DVT, nonfatal PE or death
(2.0% vs. 9.3%)
Knee RECORD3 85 Short duration rivaroxaban (10 mg daily Similar rates of major bleeding
arthroplasty 10–14 days) was superior to short duration (0.5% vs. 0.6%)
Lovenox (40 mg daily 10–14 days) at pre-
vention of any DVT, nonfatal PE or death
(9.2% vs. 18.9%)
VTE prophylaxis MAGELLAN 86 The rate of any proximal DVT or symp- The composite of major or clinically
in medically ill tomatic VTE with extended duration riva- relevant nonmajor bleeding occurred
patients roxaban (10 mg daily for 31–39 days) was more often in patients receiving
noninferior at 10 days (2.7% vs. 2.7%) and extended duration rivaroxaban at day
superior at 35 days (4.4% vs. 5.7%) when 10 (2.8% vs. 1.2%) and at day 35 (4.1%
compared to standard prophylaxis (Lovenox vs. 1.7%)
40 mg daily for 10–14 days)
(Continued )
Kaushansky_chapter 25_p0393-0408.indd 400 9/19/15 12:19 AM