Page 426 - Williams Hematology ( PDFDrive )
P. 426
400 Part V: Therapeutic Principles Chapter 25: Antithrombotic Therapy 401
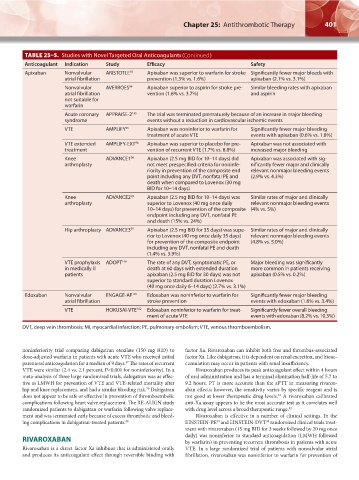
TABLE 25–5. Studies with Novel Targeted Oral Anticoagulants (Continued )
Anticoagulant Indication Study Efficacy Safety
Apixaban Nonvalvular ARISTOTLE 93 Apixaban was superior to warfarin for stroke Significantly fewer major bleeds with
atrial fibrillation prevention (1.3% vs. 1.6%) apixaban (2.1% vs. 3.1%)
Nonvalvular AVERROES 94 Apixaban superior to aspirin for stroke pre- Similar bleeding rates with apixaban
atrial fibrillation vention (1.6% vs. 3.7%) and aspirin
not suitable for
warfarin
Acute coronary APPRAISE-2 145 The trial was terminated prematurely because of an increase in major bleeding
syndrome events without a reduction in cardiovascular ischemic events
VTE AMPLIFY 95 Apixaban was noninferior to warfarin for Significantly fewer major bleeding
treatment of acute VTE events with apixaban (0.6% vs. 1.8%)
VTE extended AMPLIFY-EXT 96 Apixaban was superior to placebo for pre- Apixaban was not associated with
treatment vention of recurrent VTE (1.7% vs. 8.8%) increased major bleeding
Knee ADVANCE1 98 Apixaban (2.5 mg BID for 10–14 days) did Apixaban was associated with sig-
arthroplasty not meet prespecified criteria for noninfe- nificantly fewer major and clinically
riority in prevention of the composite end relevant nonmajor bleeding events
point including any DVT, nonfatal PE and (2.9% vs. 4.3%)
death when compared to Lovenox (30 mg
BID for 10–14 days)
Knee ADVANCE2 99 Apixaban (2.5 mg BID for 10–14 days) was Similar rates of major and clinically
arthroplasty superior to Lovenox (40 mg once daily relevant nonmajor bleeding events
10–14 days) for prevention of the composite (4% vs. 5%)
endpoint including any DVT, nonfatal PE
and death (15% vs. 24%)
Hip arthroplasty ADVANCE3 97 Apixaban (2.5 mg BID for 35 days) was supe- Similar rates of major and clinically
rior to Lovenox (40 mg once daily 35 days) relevant nonmajor bleeding events
for prevention of the composite endpoint (4.8% vs. 5.0%)
including any DVT, nonfatal PE and death
(1.4% vs. 3.9%)
VTE prophylaxis ADOPT 146 The rate of any DVT, symptomatic PE, or Major bleeding was significantly
in medically ill death at 60 days with extended duration more common in patients receiving
patients apixaban (2.5 mg BID for 30 days) was not apixaban (0.5% vs. 0.2%)
superior to standard duration Lovenox
(40 mg once daily 6–14 days) (2.7% vs. 3.1%)
Edoxaban Nonvalvular ENGAGE-AF 101 Edoxaban was noninferior to warfarin for Significantly fewer major bleeding
atrial fibrillation stroke prevention events with edoxaban (1.6% vs. 3.4%)
VTE HOKUSAI-VTE 102 Edoxaban noninferior to warfarin for treat- Significantly fewer overall bleeding
ment of acute VTE events with edoxaban (8.2% vs. 10.3%)
DVT, deep vein thrombosis; MI, myocardial infarction; PE, pulmonary embolism; VTE, venous thromboembolism.
noninferiority trial comparing dabigatran etexilate (150 mg BID) to factor Xa. Rivaroxaban can inhibit both free and thrombus-associated
dose-adjusted warfarin in patients with acute VTE who received initial factor Xa. Like dabigatran, it is dependent on renal excretion, and bioac-
parenteral anticoagulation for a median of 9 days. The rates of recurrent cumulation may occur in patients with renal insufficiency.
37
VTE were similar (2.4 vs. 2.1 percent, P<0.001 for noninferiority). In a Rivaroxaban produces its peak anticoagulant effect within 4 hours
meta-analysis of three large randomized trials, dabigatran was as effec- of oral administration and has a terminal elimination half-life of 5.7 to
tive as LMWH for prevention of VTE and VTE-related mortality after 9.2 hours. PT is more accurate than the aPTT in measuring rivarox-
hip and knee replacement, and had a similar bleeding risk. Dabigatran aban effects; however, the sensitivity varies by specific reagent and is
79
does not appear to be safe or effective in prevention of thromboembolic not good at lower therapeutic drug levels. A rivaroxaban calibrated
81
complications following heart valve replacement. The RE-ALIGN study anti-Xa assay appears to be the most accurate test as it correlates well
randomized patients to dabigatran or warfarin following valve replace- with drug level across a broad therapeutic range. 82
ment and was terminated early because of excess thrombotic and bleed- Rivaroxaban is effective in a number of clinical settings. In the
ing complications in dabigatran-treated patients. 80 EINSTEIN-PE and EINSTEIN-DVT randomized clinical trials treat-
39
38
ment with rivaroxaban (15 mg BID for 3 weeks followed by 20 mg once
RIVAROXABAN daily) was noninferior to standard anticoagulation (LMWH followed
by warfarin) in preventing recurrent thrombosis in patients with acute
Rivaroxaban is a direct factor Xa inhibitor that is administered orally VTE. In a large randomized trial of patients with nonvalvular atrial
and produces its anticoagulant effect through reversible binding with fibrillation, rivaroxaban was noninferior to warfarin for prevention of
Kaushansky_chapter 25_p0393-0408.indd 401 9/19/15 12:19 AM