Page 455 - Williams Hematology ( PDFDrive )
P. 455
430 Part V: Therapeutic Principles Chapter 28: Therapeutic Apheresis: Indications, Efficacy, and Complications 431
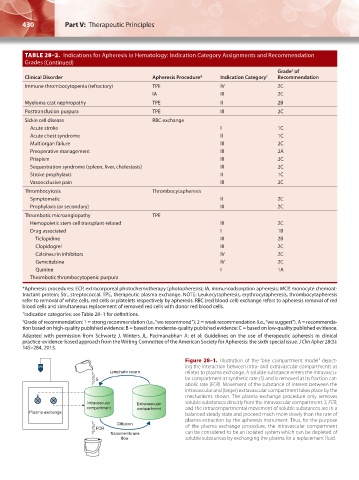
TABLE 28–2. Indications for Apheresis in Hematology: Indication Category Assignments and Recommendation
Grades (Continued)
‡
Grade of
Clinical Disorder Apheresis Procedure* Indication Category † Recommendation
Immune thrombocytopenia (refractory) TPE IV 2C
IA III 2C
Myeloma cast nephropathy TPE II 2B
Posttransfusion purpura TPE III 2C
Sickle cell disease RBC exchange
Acute stroke I 1C
Acute chest syndrome II 1C
Multiorgan failure III 2C
Preoperative management III 2A
Priapism III 2C
Sequestration syndrome (spleen, liver, cholestasis) III 2C
Stroke prophylaxis II 1C
Vasoocclusive pain III 2C
Thrombocytosis Thrombocytapheresis
Symptomatic II 2C
Prophylaxis (or secondary) III 2C
Thrombotic microangiopathy TPE
Hemopoietic stem cell transplant-related III 2C
Drug associated I 1B
Ticlopidine III 2B
Clopidogrel III 2C
Calcineurin inhibitors IV 2C
Gemcitabine IV 2C
Quinine I 1A
Thrombotic thrombocytopenic purpura
*Apheresis procedures: ECP, extracorporeal photochemotherapy (photopheresis); IA, immunoadsorption apheresis; MCP, monocyte chemoat-
tractant protein; Str., streptococcal. TPE, therapeutic plasma exchange. NOTE: Leukocytapheresis, erythrocytapheresis, thrombocytapheresis
refer to removal of white cells, red cells or platelets respectively by apheresis. RBC (red blood cell) exchange refers to apheresis removal of red
blood cells and simultaneous replacement of removed red cells with donor red blood cells.
† Indication categories: see Table 28–1 for definitions.
‡ Grade of recommendation: 1 = strong recommendation (i.e., “we recommend”); 2 = weak recommendation (i.e., “we suggest”). A = recommenda-
tion based on high-quality published evidence; B = based on moderate-quality published evidence; C = based on low-quality published evidence.
Adapted with permission from Schwartz J, Winters JL, Padmanabhan A: et al: Guidelines on the use of therapeutic apheresis in clinical
practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher 28(3):
145–284, 2013.
Figure 28–1. Illustration of the “one compartment model” depict-
ing the interaction between intra- and extravascular compartments as
Lymphatic return relates to plasma exchange. A soluble substance enters the intravascu-
S lar compartment at synthetic rate (S) and is removed at its fraction cat-
abolic rate (FCR). Movement of the substance of interest between the
intravascular and (larger) extravascular compartment takes place by the
mechanisms shown. The plasma exchange procedure only removes
Intravascular Extravascular soluble substances directly from the intravascular compartment. S, FCR,
compartment compartment and the intracompartmental movement of soluble substances are in a
Plasma exchange balanced steady state and proceed much more slowly than the rate of
plasma extraction by the apheresis instrument. Thus, for the purpose
Diffusion of the plasma exchange procedure, the intravascular compartment
FCR
Transmembrane can be considered to be an isolated system which can be depleted of
flow soluble substances by exchanging the plasma for a replacement fluid.
Kaushansky_chapter 28_p0427-0436.indd 430 9/17/15 6:05 PM