Page 456 - Williams Hematology ( PDFDrive )
P. 456
430 Part V: Therapeutic Principles Chapter 28: Therapeutic Apheresis: Indications, Efficacy, and Complications 431
100 RED CELL APHERESIS
Red cell exchange refers to the removal of a patient’s red cells in exchange
80 y = y e –x for donor red cells. When red cells are removed for therapeutic pur-
0
t
Percent remaining (y t ) 60 erythrocytapheresis. Red cell exchange is employed in settings where a
poses, but not replaced with donor red cells, the process is referred to as
clinical disorder is caused by an abnormality (inherited or acquired) of
the patient’s red blood cells, and erythrocytapheresis may be employed
40
in situations characterized by an untoward elevation in circulating red
cell volume or in iron-overload states.
48
20
RED CELL EXCHANGE
0
Therapeutic red cell exchange can be performed manually (i.e.,
“exchange transfusion”) or by apheresis using automated programma-
0.0 0.5 1.0 1.5 2.0 2.5 3.0 ble blood-processing equipment. 49–51 This discussion focuses on auto-
Plasma volumes processed (x) mated red cell exchange. The programming functions of automated
apheresis instruments are used to determine the parameters of red cell
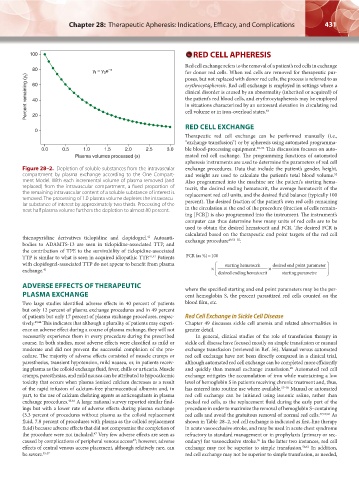
Figure 28–2. Depletion of soluble substances from the intravascular exchange procedures. Data that include the patient’s gender, height,
compartment by plasma exchange according to the One Compart- and weight are used to calculate the patient’s total blood volume.
52
ment Model. With each incremental volume of plasma removed (and Also programmed into the machine are the patient’s starting hema-
replaced) from the intravascular compartment, a fixed proportion of tocrit, the desired ending hematocrit, the average hematocrit of the
the remaining intravascular content of a soluble substance of interest is replacement red cell units, and the desired fluid balance (typically 100
removed. The processing of 1.0 plasma volume depletes the intravascu- percent). The desired fraction of the patient’s own red cells remaining
lar substance of interest by approximately two thirds. Processing of the in the circulation at the end of the procedure (fraction of cells remain-
next half plasma volume furthers the depletion to almost 80 percent.
ing [FCR]) is also programmed into the instrument. The instrument’s
computer can thus determine how many units of red cells are to be
used to obtain the desired hematocrit and FCR. The desired FCR is
calculated based on the therapeutic end point targets of the red cell
thienopyridine derivatives ticlopidine and clopidogrel. Autoanti- exchange procedure 49,53–55 :
41
bodies to ADAMTS-13 are seen in ticlopidine-associated TTP, and
the contribution of TPE to the survivability of ticlopidine-associated
=
TTP is similar to what is seen in acquired idiopathic TTP. 41,42 Patients FCR(as %) 100
with clopidogrel-associated TTP do not appear to benefit from plasma starting hematocrit desiredend pointparameter
exchange. 41 × desiredendinghematocrit × starting parametre
ADVERSE EFFECTS OF THERAPEUTIC where the specified starting and end point parameters may be the per-
PLASMA EXCHANGE cent hemoglobin S, the percent parasitized red cells counted on the
Two large studies identified adverse effects in 40 percent of patients blood film, etc.
but only 12 percent of plasma exchange procedures and in 49 percent
of patients but only 17 percent of plasma exchange procedures, respec- Red Cell Exchange in Sickle Cell Disease
tively. 43,44 This indicates that although a plurality of patients may experi- Chapter 49 discusses sickle cell anemia and related abnormalities in
ence an adverse effect during a course of plasma exchange, they will not greater detail.
necessarily experience them in every procedure during the prescribed In general, clinical studies of the role of transfusion therapy in
course. In both studies, most adverse effects were classified as mild or sickle cell disease have focused mostly on simple transfusion or manual
moderate and did not prevent the successful completion of the pro- exchange transfusion (reviewed in Ref. 56). Manual versus automated
cedure. The majority of adverse effects consisted of muscle cramps or red cell exchange have not been directly compared in a clinical trial,
paresthesias, transient hypotension, mild nausea, or, in patients receiv- although automated red cell exchange can be completed more efficiently
ing plasma as the colloid exchange fluid, fever, chills or urticaria. Muscle and quickly than manual exchange transfusion. Automated red cell
49
cramps, paresthesias, and mild nausea can be attributed to hypocalcemic exchange mitigates the accumulation of iron while maintaining a low
toxicity that occurs when plasma ionized calcium decreases as a result level of hemoglobin S in patients receiving chronic treatment and, thus,
of the rapid infusion of calcium-free pharmaceutical albumin and, in has entered into routine use where available. 57–59 Manual or automated
part, to the use of calcium chelating agents as anticoagulants in plasma red cell exchange can be initiated using isotonic saline, rather than
exchange procedures. 45,46 A large national survey reported similar find- packed red cells, as the replacement fluid during the early part of the
ings but with a lower rate of adverse effects during plasma exchange procedure in order to maximize the removal of hemoglobin S–containing
(3.3 percent of procedures without plasma as the colloid replacement red cells and avoid the gratuitous removal of normal red cells. 57,59,60 As
fluid, 7.8 percent of procedures with plasma as the colloid replacement shown in Table 28–2, red cell exchange is indicated as first-line therapy
fluid) because adverse effects that did not compromise the completion of in acute vasoocclusive stroke, and may be used in acute chest syndrome
the procedure were not included. Very few adverse effects are seen as refractory to standard management or in prophylaxis (primary or sec-
47
caused by complications of peripheral venous access ; however, adverse ondary) for vasoocclusive stroke. In the latter two instances, red cell
47
56
effects of central venous access placement, although relatively rare, can exchange may not be superior to simple transfusion. 56,61 In addition,
be severe. 45,47 red cell exchange may not be superior to simple transfusion, as needed,
Kaushansky_chapter 28_p0427-0436.indd 431 9/17/15 6:05 PM