Page 507 - Williams Hematology ( PDFDrive )
P. 507
482 Part VI: The Erythrocyte Chapter 32: Erythropoiesis 483
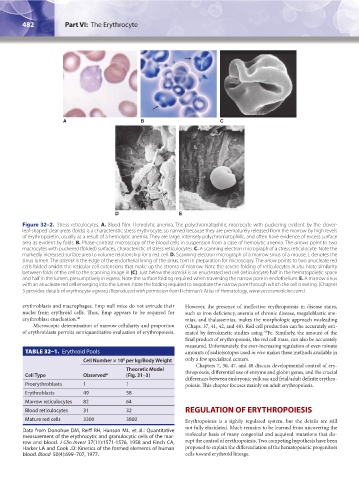
A B C
∗ L L
L L
D E
Figure 32–2. Stress reticulocytes. A. Blood film. Hemolytic anemia. The polychromatophilic macrocyte with puckering evident by the clover-
leaf-shaped clear areas (folds) is a characteristic stress erythrocyte, so named because they are prematurely released from the marrow by high levels
of erythropoietin, usually as a result of a hemolytic anemia. They are large, intensely polychromatophilic, and often have evidence of excess surface
area as evident by folds. B. Phase-contrast microscopy of the blood cells in suspension from a case of hemolytic anemia. The arrows point to two
macrocytes with puckered (folded) surfaces, characteristic of stress reticulocytes. C. A scanning electron micrograph of a stress reticulocyte. Note the
markedly increased surface area to volume relationship for a red cell. D. Scanning electron micrograph of a marrow sinus of a mouse. L denotes the
sinus lumen. The asterisk is the edge of the endothelial lining of the sinus, torn in preparation for microscopy. The arrow points to two anucleate red
cells folded amidst the reticular cell extensions that make up the stroma of marrow. Note the severe folding of reticulocytes in situ. Note similarity
between folds of the cell to the scanning image in (C). Just below the asterisk is an enucleated red cell (reticulocyte) half in the hematopoietic space
and half in the lumen, presumptively in egress. Note the surface folding required when traversing the narrow pore in endothelium. E. A marrow sinus
with an anucleate red cell emerging into the lumen. Note the folding required to negotiate the narrow pore through which the cell is exiting. (Chapter
3 provides details of erythrocyte egress.) (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
erythroblasts and macrophages. Emp null mice do not extrude their However, the presence of ineffective erythropoiesis in disease states,
nuclei from erythroid cells. Thus, Emp appears to be required for such as iron deficiency, anemia of chronic disease, megaloblastic ane-
erythroblast enucleation. 48 mias, and thalassemias, makes the morphologic approach misleading
Microscopic determination of marrow cellularity and proportion (Chaps. 37, 41, 42, and 48). Red cell production can be accurately esti-
of erythroblasts permits semiquantitative evaluation of erythropoiesis. mated by ferrokinetic studies using Fe. Similarly, the amount of the
59
final product of erythropoiesis, the red cell mass, can also be accurately
measured. Unfortunately, the ever-increasing regulation of even minute
TABLE 32–1. Erythroid Pools amounts of radioisotopes used in vivo makes these methods available in
Cell Number × 10 per kg/Body Weight only a few specialized centers.
8
Chapters 7, 30, 47, and 48 discuss developmental control of ery-
Theoretic Model
Cell Type Observed* (Fig. 31–3) thropoiesis, differential use of enzyme and globin genes, and the crucial
differences between embryonic yolk sac and fetal/adult definite erythro-
Proerythroblasts 1 1 poiesis. This chapter focuses mainly on adult erythropoiesis.
Erythroblasts 49 58
Marrow reticulocytes 82 64
Blood reticulocytes 31 32 REGULATION OF ERYTHROPOIESIS
Mature red cells 3300 3800 Erythropoiesis is a tightly regulated system, but the details are still
not fully elucidated. Much remains to be learned from uncovering the
Data from Donohue DM, Reiff RH, Hanson ML, et al.: Quantitative
measurement of the erythrocytic and granulocytic cells of the mar- molecular basis of many congenital and acquired mutations that dis-
row and blood. J Clin Invest 37(11):1571-1576, 1958 and Finch CA, rupt the control of erythropoiesis. Two competing hypothesis have been
Harker LA and Cook JD: Kinetics of the formed elements of human proposed to explain the differentiation of the hematopoietic progenitors
blood. Blood 50(4):699–707, 1977. cells toward erythroid lineage.
Kaushansky_chapter 32_p0479-0494.indd 482 9/17/15 6:10 PM