Page 510 - Williams Hematology ( PDFDrive )
P. 510
484 Part VI: The Erythrocyte Chapter 32: Erythropoiesis 485
Runx1
LT-HSC Scl/tal1 Stem cell class
Pluripotent stem cells Self- Bmi-1 Required for production,
Lmo2
MII
survival, or self-renewal
renewal
Tel
of HSCs
Gfi-1
GATA-2
ST-HSC
CMP CLP
Multipotent progenitors MEP GMP Ikaros
PU.1
Notch
PU.1 E2A TCF-1
GATA-1 EBF
GATA-2 C/EBPα Pax5 GATA-3
FOG1 GATA-2 GATA-1 Bcl11a
(Evi9)
Committed precursors GATA-1 GATA-1 Gfi-1
FOG1
Gfi-1b
EKLF Gfi-1b GATA-1 C/EBPc XBP-1
Mature cells RBC Megakaryocyte Mast cell Eosinophil Neutrophil Macrophage B lymphocyte T lymphocyte
Monocyte/
LT-HSC: long term HSCs
Fli-1
ST HSC: short term HSCs
Nf-E2
CMP: Common myeloid progenitor
CLP: common lymphoid progenitor
MEP: megakaryocyte/erythroid progenitors
Platelets GMP: granulocyte/macrophage progenitors
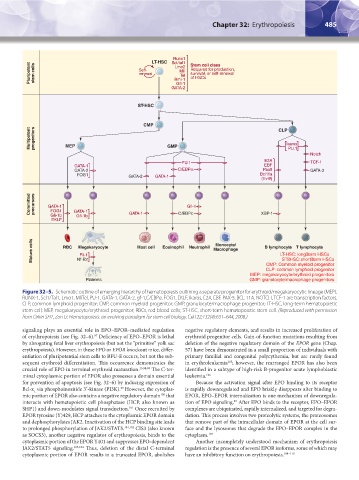
Figure 32–5. Schematic outline of emerging hierarchy of hematopoiesis outlining a separate progenitor for erythroid/megakaryocytic lineage (MEP).
RUNX-1, Scl1/Tal1, Lmo1, MllTel, PU-1, GATA-1, GATA-2, gf-1,C/EBPα, FOG1, EKLF, Ikaros, E2A, EBF, PAX-5, BCL 11A, NOTCH, TCF-1 are transcription factors.
CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte/macrophage progenitor; LT-HSC, long-term hematopoietic
stem cell; MEP, megakaryocyte/erythroid progenitor; RBCs, red blood cells; ST-HSC, short-term hematopoietic stem cell. (Reproduced with permission
from Orkin SH1, Zon LI: Hematopoiesis: an evolving paradigm for stem cell biology. Cell 22;132(4):631–644, 2008.)
signaling plays an essential role in EPO–EPOR–mediated regulation negative regulatory elements, and results in increased proliferation of
of erythropoiesis (see Fig. 32–6). Deficiency of EPO–EPOR is lethal erythroid progenitor cells. Gain-of-function mutations resulting from
97
by abrogating fetal liver erythropoiesis (but not the “primitive” yolk sac deletion of the negative regulatory domain of the EPOR gene (Chap.
erythropoiesis). However, in these EPO or EPOR knockout mice, differ- 57) have been demonstrated in a small proportion of individuals with
entiation of pluripotential stem cells to BFU-E occurs, but not the sub- primary familial and congenital polycythemia, but are rarely found
sequent erythroid differentiation. This occurrence demonstrates the in erythroleukemia ; however, the rearranged EPOR has also been
105
crucial role of EPO in terminal erythroid maturation. 35,98,99 The C-ter- identified in a subtype of high-risk B-progenitor acute lymphoblastic
minal cytoplasmic portion of EPOR also possesses a domain essential leukemia. 106
for prevention of apoptosis (see Fig. 32–6) by inducing expression of Because the activation signal after EPO binding to its receptor
Bcl-x via phosphoinositide 3′-kinase (PI3K). However, the cytoplas- is rapidly downregulated and EPO briskly disappears after binding to
53
L
mic portion of EPOR also contains a negative regulatory domain that EPOR, EPO–EPOR internalization is one mechanism of downregula-
100
interacts with hematopoietic cell phosphatase (HCP, also known as tion of EPO signaling. After EPO binds to the receptor, EPO–EPOR
92
SHP1) and down-modulates signal transduction. Once recruited by complexes are ubiquinated, rapidly internalized, and targeted for degra-
101
EPOR tyrosine (Y)429, HCP attaches to the cytoplasmic EPOR domain dation. This process involves two proteolytic systems, the proteosomes
and dephosphorylates JAK2. Inactivation of the HCP binding site leads that remove part of the intracellular domain of EPOR at the cell sur-
to prolonged phosphorylation of JAK2/STAT5. 101,102 CIS3 (also known face and the lysosomes that degrade the EPO–EPOR complex in the
as SOCS3), another negative regulator of erythropoiesis, binds to the cytoplasm. 107
cytoplasmic portion of the EPOR Y401 and suppresses EPO-dependent Another incompletely understood mechanism of erythropoiesis
JAK2/STAT5 signaling. 103,104 Thus, deletion of the distal C-terminal regulation is the presence of several EPOR isoforms, some of which may
cytoplasmic portion of EPOR results in a truncated EPOR, abolishes have an inhibitory function on erythropoiesis. 108–110
Kaushansky_chapter 32_p0479-0494.indd 485 9/17/15 6:10 PM