Page 505 - Williams Hematology ( PDFDrive )
P. 505
480 Part VI: The Erythrocyte Chapter 32: Erythropoiesis 481
more advanced phylum Annelida. However, the evolutionary advantage ceases, and erythropoiesis moves to the marrow (Chaps. 7 and 48 pro-
derived from enucleation appears to be slight. Nucleated red cells are vide details of developmental switching of embryonic, fetal, and adult
observed in more advanced animals, such as reptiles and birds. All globin expression).
14
mammalian erythrocytes are nonnucleated and in most species are disc During the neonatal period, the volume of available marrow
15
shaped, but are oval in some species. Enucleation decreases the work- space is almost the same as the total volume of hematopoietic cells and
28
load of heart as it reduces one third of the cell weight. marrow vasculature. This process continues for a few years until the
In nonmammalian species, the spleen is the fundamental erythro- growth of bones and bone cavities exceeds the growth of hematopoi-
poietic organ. However, in some fish, the kidneys also are involved in etic mass. However, whenever the demand on erythropoiesis increases
red cell production. 16,17 In vertebrates, an evolutionary shift occurred (blood loss, hypoxia, ineffective erythropoiesis, or hemolysis), the lack
18
from the spleen to the liver and from the liver to the bones cavities. of reserve space in neonates and small children reactivates extramedul-
29
The homeostatic regulation of blood or hemoglobin production has lary erythropoiesis in the liver and spleen. In adults, expansion of mar-
8
been studied in Daphnia, where a balance exists between oxygen need row space continues, and the amount of fatty tissue gradually increases
and hemoglobin production. In higher animals, this relationship is in all bone cavities. Because of the abundant marrow space, compen-
maintained by adjusting red cell production. Studies of birds, fish, satory reactivation of extramedullary sites rarely occurs in later life.
19
20
21
and mammals indicate that red cell production is controlled by EPO, Extramedullary hematopoiesis during adult years indicates pathologic
which is capable of adjusting red cell production to the demands for rather than compensatory blood formation, such as seen in primary
oxygen in the tissues. EPO of mammals has considerable biologic simi- myelofibrosis (Chap. 86) wherein the stem cells have abnormal inter-
30
larity and genetic homology. 22 action with the extracellular matrix. During fetal life, EPO production
31
is primarily hepatic. At birth, a gradual switch to renal production of
EPO occurs. In the adult, the kidney is responsible for approximately
ONTOGENY OF RED CELL PRODUCTION 85 percent of total production. 32,33
EMBRYONIC AND FETAL ERYTHROPOIESIS CELLULAR COMPONENTS OF
The environment within the bone apparently is optimal for cellular
proliferation and maturation. However, bone cavities do not develop ERYTHROPOIESIS
until the fifth fetal month. Other, presumably less favorable, sites are
responsible for red cell production during early embryonic life (Chap. PROGENITOR CELLS
7). In the human, large nucleated blood cells are first formed in the yolk Our ability to evaluate early erythropoiesis rests on functional assays
sac, and some enucleate. They cluster in blood islands that become of hematopoietic progenitors. The developmentally earliest progenitor
23
24
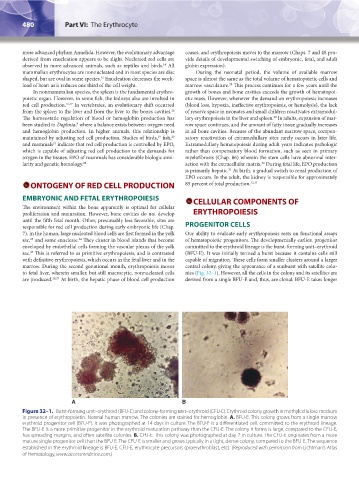
enveloped by endothelial cells forming the vascular plexus of the yolk committed to the erythroid lineage is the burst-forming unit–erythroid
sac. This is referred to as primitive erythropoiesis, and is contrasted (BFU-E). It was initially termed a burst because it contains cells still
25
with definitive erythropoiesis, which occurs in the fetal liver and in the capable of migration. These cells form smaller clusters around a larger
marrow. During the second gestational month, erythropoiesis moves central colony, giving the appearance of a sunburst with satellite colo-
to fetal liver, wherein smaller, but still macrocytic, nonnucleated cells nies (Fig. 32–1). However, all the cells in the colony and its satellites are
are produced. 26,27 At birth, the hepatic phase of blood cell production derived from a single BFU-E and, thus, are clonal. BFU-E takes longer
A B
Figure 32–1. Burst-forming unit–erythroid (BFU-E) and colony-forming unit–erythroid (CFU-E). Erythroid colony growth in methylcellulose medium
in presence of erythropoietin. Normal human marrow. The colonies are stained for hemoglobin. A. BFU-E. This colony grows from a single marrow
erythroid progenitor cell (BFU-E). It was photographed at 14 days in culture. The BFU-E is a differentiated cell, committed to the erythroid lineage.
The BFU-E is a more primitive progenitor in the erythroid maturation pathway than the CFU-E. The colony it forms is large, compared to the CFU-E,
has spreading margins, and often satellite colonies. B. CFU-E. This colony was photographed at day 7 in culture. The CFU-E originates from a more
mature single progenitor cell than the BFU-E. The CFU-E is smaller and grows typically in a tight, dense colony, compared to the BFU-E. The sequence
established in the erythroid lineage is BFU-E, CFU-E, erythrocyte precursors (proerythroblast, etc). (Reproduced with permission from Lichtman’s Atlas
of Hematology, www.accessmedicine.com.)
Kaushansky_chapter 32_p0479-0494.indd 480 9/17/15 6:10 PM