Page 576 - Williams Hematology ( PDFDrive )
P. 576
550 Part VI: The Erythrocyte Chapter 37: Anemia of Chronic Disease 551
bacterial lipopolysaccharide or IL-1β to mimic a septic state. However, macrophage recycling of senescent erythrocytes and from hepatocyte
24
suppression of EPO production is not the major mechanism of AI. If iron stores; only approximately 1 to 2 mg come from dietary iron. Only
it were, administration of relatively small amounts of EPO should be approximately 2 to 4 mg of iron is bound to transferrin but the entire
sufficient to reverse the AI. daily iron flow transits through this compartment; thus, the iron in this
In contrast, relative EPO deficiency is often a major contributor pool turns over every few hours. During inflammation the release of
to anemia of CKD. Most destructive diseases affecting the kidneys also iron from macrophages and probably also from liver stores is markedly
decrease the release of EPO. 25,26 In the kidney, interstitial fibroblasts of inhibited. 39–45 Studies in transgenic mice lacking hepcidin and mice
neural crest origin 26,27 are probably the main source of EPO, but the overexpressing hepcidin indicate that the peptide is a negative regula-
identity of EPO-producing cells in the kidney remains controversial, tor of iron release from macrophages and of intestinal iron uptake. 46,47
mostly because the basal production of EPO is very low and ultrasen- During inflammation, IL-6 induces hepcidin production, which in turn
sitive methods are required to detect the source of the hormone. In inhibits iron release from macrophages (and probably from hepatoc-
response to anemia or hypoxia, the number of renal cells producing EPO ytes), leading to hypoferremia (Fig. 37–2). Hepcidin acts by binding
increases. In advanced CKD, the kidneys undergo end-stage fibrosis, to cell membrane-associated ferroportin molecules that are the only
during which these fibroblasts may transdifferentiate into myofibrob- conduits for iron export, and inducing ferroportin internalization and
48
lasts and lose their ability to produce appropriate amounts of EPO in degradation. As hepcidin concentrations increase, less and less ferro-
response to hypoxia. 26,27 However, these or other renal cells can be acti- portin is available for iron export and the iron release into plasma from
vated to increase their EPO output by the administration of therapeu- macrophages, hepatocytes, and enterocytes decreases.
28
tic prolyl-hydroxylase inhibitors (Chap. 32), as indicated by the lower
stimulated EPO production by anephric patients compared to those Erythropoiesis in Anemia of Inflammation Is Limited by Iron
with end-stage renal disease and retained kidneys. Studies in animal As an intermediate step during the synthesis of heme, iron becomes
models indicate that the impairment of EPO production in end-stage incorporated into protoporphyrin IX. Zinc is an alternative protopor-
kidneys may be reversible and could be therapeutically restored. 26,27 phyrin ligand. In iron deficiency, increased amounts of zinc are incorpo-
Inflammation is also a strong contributor to the pathogenesis of rated into protoporphyrin. In AI, zinc protoporphyrin is also increased.
49
anemia of CKD. Patients who had renal disease with inflammation, as
measured by increased serum CRP greater than 20 mg/L, required on
the average 80 percent higher doses of EPO than patients with simple Infection, inflammatory
primary EPO deficiency from renal disease. In another study, patients stimulus
29
with CRP greater than 50 mg/L reached lower concentrations of Hgb
than patients with CRP less than 50 mg/L, despite higher doses of Senescent RBC
erythropoiesis-stimulating agents. Inflammation thus induces a state
30
of relative resistance to EPO, contributing to the pathogenesis of anemia
of CKD.
Liver
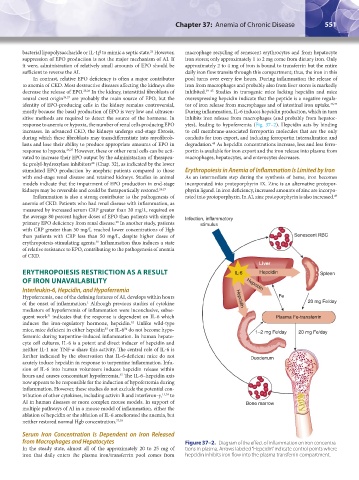
ERYTHROPOIESIS RESTRICTION AS A RESULT IL-6 Hepcidin Spleen
OF IRON UNAVAILABILITY Hepcidin
Interleukin-6, Hepcidin, and Hypoferremia
Hypoferremia, one of the defining features of AI, develops within hours Hepcidin Fe
1
of the onset of inflammation. Although previous studies of cytokine 20 mg Fe/day
mediators of hypoferremia of inflammation were inconclusive, subse-
31
quent work indicates that the response is dependent on IL-6 which Plasma Fe-transferrin
induces the iron-regulatory hormone, hepcidin. Unlike wild-type
32
mice, mice deficient in either hepcidin or IL-6 do not become hypo- 1–2 mg Fe/day 20 mg Fe/day
34
33
ferremic during turpentine-induced inflammation. In human hepato-
cyte cell cultures, IL-6 is a potent and direct inducer of hepcidin and
neither IL-1 nor TNF-α share this activity. The central role of IL-6 is
further indicated by the observation that IL-6-deficient mice do not Duodenum
acutely induce hepcidin in response to turpentine inflammation. Infu-
sion of IL-6 into human volunteers induces hepcidin release within
35
hours and causes concomitant hypoferremia. The IL-6–hepcidin axis
now appears to be responsible for the induction of hypoferremia during
inflammation. However, these studies do not exclude the potential con-
tribution of other cytokines, including activin B and interferon-γ, 13,36 to
AI in human diseases or more complex mouse models. In support of Bone marrow
multiple pathways of AI in a mouse model of inflammation, either the
ablation of hepcidin or the ablation of IL-6 ameliorated the anemia, but
neither restored normal Hgb concentration. 37,38
Serum Iron Concentration Is Dependent on Iron Released
from Macrophages and Hepatocytes Figure 37–2. Diagram of the effect of inflammation on iron concentra-
In the steady state, almost all of the approximately 20 to 25 mg of tions in plasma. Arrows labeled “Hepcidin” indicate control points where
iron that daily enters the plasma iron/transferrin pool comes from hepcidin inhibits iron flow into the plasma transferrin compartment.
Kaushansky_chapter 37_p0549-0558.indd 551 9/17/15 6:16 PM