Page 575 - Williams Hematology ( PDFDrive )
P. 575
550 Part VI: The Erythrocyte Chapter 37: Anemia of Chronic Disease 551
50 ETIOLOGY AND PATHOGENESIS
In the chronic setting, AI predominantly results from the body’s inabil-
40 ity to increase erythrocyte production to compensate for relatively small
decrements in erythrocyte survival (reviewed in Ref. 1). In the steady
state, erythrocyte production is sufficiently high so that the resulting
30 anemia is mild to moderate. The anemia associated with acute critical
Hct (%) illness has the same pathogenesis as other forms of AI but it devel-
20 ops more rapidly perhaps because of the more extensive erythrocyte
destruction and intensive diagnostic phlebotomy common in this set-
ting. The key questions about the pathogenesis of AI, still only partially
10 answered, are: (1) What accounts for the inability of the AI marrow to
increase erythropoiesis? and (2) How is this deficit connected to the
characteristic hypoferremia and sequestration of iron in macrophages
0 20 40 60 80 100 and hepatocytes? Anemia of CKD is similar to AI but the underly-
2
Creatinine clearance (mL/min/1.73 m ) ing renal pathology also impairs the ability of the kidneys to produce
enough EPO leading to insufficient compensatory erythropoiesis.
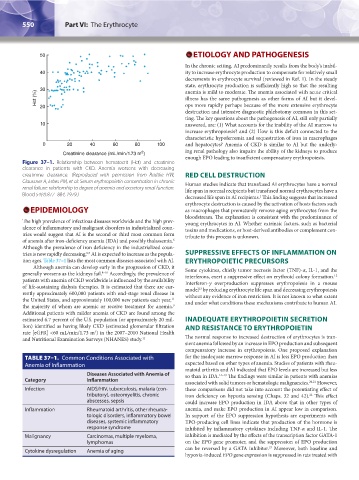
Figure 37–1. Relationship between hematocrit (Hct) and creatinine
clearance in patients with CKD. Anemia worsens with decreasing
creatinine clearance. (Reproduced with permission from Radtke HW, RED CELL DESTRUCTION
Claussner A, Erbes PM, et al: Serum erythropoietin concentration in chronic Human studies indicate that transfused AI erythrocytes have a normal
renal failure: relationship to degree of anemia and excretory renal function. life span in normal recipients but transfused normal erythrocytes have a
Blood 54(4):877–884, 1979.) decreased life span in AI recipients. This finding suggests that increased
1
erythrocyte destruction is caused by the activation of hosts factors such
EPIDEMIOLOGY as macrophages that prematurely remove aging erythrocytes from the
bloodstream. The explanation is consistent with the predominance of
The high prevalence of infectious diseases worldwide and the high prev- young erythrocytes in AI. Whether extrinsic factors, such as bacterial
alence of inflammatory and malignant disorders in industrialized coun- toxins and medications, or host-derived antibodies or complement con-
tries would suggest that AI is the second or third most common form tribute to this process is unknown.
of anemia after iron-deficiency anemia (IDA) and possibly thalassemia. 6
Although the prevalence of iron deficiency in the industrialized coun-
tries is now rapidly decreasing, AI is expected to increase as the popula- SUPPRESSIVE EFFECTS OF INFLAMMATION ON
6,7
tion ages. Table 37–1 lists the most common diseases associated with AI. ERYTHROPOIETIC PRECURSORS
Although anemia can develop early in the progression of CKD, it Some cytokines, chiefly tumor necrosis factor (TNF)-α, IL-1, and the
generally worsens as the kidneys fail. 8–10 Accordingly, the prevalence of interferons, exert a suppressive effect on erythroid colony formation.
12
patients with anemia of CKD worldwide is influenced by the availability Interferon-γ overproduction suppresses erythropoiesis in a mouse
of life-sustaining dialysis therapies. It is estimated that there are cur- model by reducing erythrocyte life span and decreasing erythropoiesis
13
rently approximately 600,000 patients with end-stage renal disease in without any evidence of iron restriction. It is not known to what extent
11
the United States, and approximately 100,000 new patients each year, and under what conditions these mechanisms contribute to human AI.
the majority of whom are anemic or receive treatment for anemia. 9
Additional patients with milder anemia of CKD are found among the
estimated 6.7 percent of the U.S. population (or approximately 20 mil- INADEQUATE ERYTHROPOIETIN SECRETION
lion) identified as having likely CKD (estimated glomerular filtration AND RESISTANCE TO ERYTHROPOIETIN
2
rate [eGFR] <60 mL/min/1.73 m ) in the 2007–2010 National Health
and Nutritional Examination Surveys (NHANES) study. 11 The normal response to increased destruction of erythrocytes is tran-
sient anemia followed by an increase in EPO production and subsequent
compensatory increase in erythropoiesis. One proposed explanation
TABLE 37–1. Common Conditions Associated with for the inadequate marrow response in AI is less EPO production than
Anemia of Inflammation expected based on other types of anemia. Studies of patients with rheu-
matoid arthritis and AI indicated that EPO levels are increased but less
Diseases Associated with Anemia of so than in IDA. 14–19 The findings were similar in patients with anemias
Category Inflammation associated with solid tumors or hematologic malignancies. 20,21 However,
Infection AIDS/HIV, tuberculosis, malaria (con- these comparisons did not take into account the potentiating effect of
tributory), osteomyelitis, chronic iron deficiency on hypoxia sensing (Chaps. 32 and 42). This effect
22
abscesses, sepsis could increase EPO production in IDA above that in other types of
Inflammation Rheumatoid arthritis, other rheuma- anemia, and make EPO production in AI appear low in comparison.
tologic disorders, inflammatory bowel In support of the EPO suppression hypothesis are experiments with
diseases, systemic inflammatory EPO-producing cell lines indicate that production of the hormone is
response syndrome inhibited by inflammatory cytokines including TNF-α and IL-1. The
Malignancy Carcinomas, multiple myeloma, inhibition is mediated by the effects of the transcription factor GATA-1
lymphomas on the EPO gene promoter, and the suppression of EPO production
can be reversed by a GATA inhibitor. Moreover, both baseline and
23
Cytokine dysregulation Anemia of aging
hypoxia-induced EPO gene expression is suppressed in rats treated with
Kaushansky_chapter 37_p0549-0558.indd 550 9/17/15 6:16 PM