Page 590 - Williams Hematology ( PDFDrive )
P. 590
564 Part VI: The Erythrocyte Chapter 39: The Congenital Dyserythropoietic Anemias 565
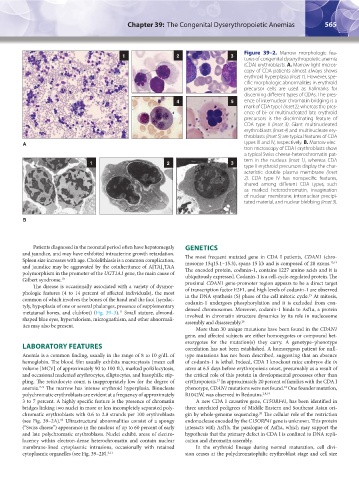
Figure 39–2. Marrow morphologic fea-
1 2 3
tures of congenital dyserythropoietic anemia
(CDA) erythroblasts. A. Marrow light micros-
copy of CDA patients almost always shows
erythroid hyperplasia (inset 1). However, spe-
cific morphologic abnormalities in erythroid
precursor cells are used as hallmarks for
discerning different types of CDAs. The pres-
4 5 ence of internuclear chromatin bridging is a
mark of CDA type I (inset 2), whereas the pres-
ence of bi- or multinucleated late erythroid
precursors is the discriminating feature of
CDA type II (inset 3). Giant multinucleated
erythroblasts (Inset 4) and multinucleate ery-
throblasts (Inset 5) are typical features of CDA
A types III and IV, respectively. B. Marrow elec-
tron microscopy of CDA I erythroblasts show
a typical Swiss cheese-heterochromatin pat-
tern in the nucleus (inset 1), whereas CDA
1 2 3
type II erythroid precursors display the char-
acteristic double plasma membrane (inset
2). CDA type IV has nonspecific features,
shared among different CDA types, such
as marked heterochromatin, invagination
of nuclear membrane, intranuclear precipi-
tated material, and nuclear blebbing (inset 3).
B
Patients diagnosed in the neonatal period often have hepatomegaly GENETICS
and jaundice, and may have exhibited intrauterine growth retardation. The most frequent mutated gene in CDA I patients, CDAN1 (chro-
Spleen size increases with age. Cholelithiasis is a common complication, mosome 15q15.1–15.3), spans 15 kb and is composed of 28 exons. 13,14
and jaundice may be aggravated by the coinheritance of A[TA] TAA The encoded protein, codanin-1, contains 1227 amino acids and it is
7
polymorphism in the promoter of the UGT1A1 gene, the main cause of ubiquitously expressed. Codanin-1 is a cell-cycle-regulated protein. The
Gilbert syndrome. 10 proximal CDAN1 gene-promoter region appears to be a direct target
The disease is occasionally associated with a variety of dysmor- of transcription factor E2F1, and high levels of codanin-1 are observed
phologic features (4 to 14 percent of affected individuals), the most in the DNA synthesis (S) phase of the cell mitotic cycle. At mitosis,
15
common of which involves the bones of the hand and the foot (syndac- codanin-1 undergoes phosphorylation and it is excluded from con-
tyly, hypoplasia of one or several phalanges, presence of supplementary densed chromosomes. Moreover, codanin-1 binds to Asf1a, a protein
metatarsal bones, and clubfoot) (Fig. 39–3). Small stature, almond- involved in chromatin structure dynamics by its role in nucleosome
11
shaped blue eyes, hypertelorism, micrognathism, and other abnormali- assembly and disassembly. 16
ties may also be present.
More than 30 unique mutations have been found in the CDAN1
gene, and affected subjects are either homozygotes or compound het-
erozygotes for the mutation(s) they carry. A genotype–phenotype
LABORATORY FEATURES correlation has not been established. A homozygous patient for null-
Anemia is a common finding, usually in the range of 8 to 10 g/dL of type mutations has not been described, suggesting that an absence
hemoglobin. The blood film usually exhibits macrocytosis (mean cell of codanin-1 is lethal. Indeed, CDA I knockout mice embryos die in
volume [MCV] of approximately 90 to 100 fL), marked poikilocytosis, utero at 6.5 days before erythropoiesis onset, presumably as a result of
and occasional nucleated erythrocytes, elliptocytes, and basophilic stip- the critical role of this protein in developmental processes other than
pling. The reticulocyte count is inappropriately low for the degree of erythropoiesis. In approximately 20 percent of families with the CDA I
17
anemia. The marrow has intense erythroid hyperplasia. Binucleate phenotype, CDAN1 mutations were not found. One founder mutation,
18
7–9
polychromatic erythroblasts are evident at a frequency of approximately R1042W, was observed in Bedouins. 2,8,19
3 to 7 percent. A highly specific feature is the presence of chromatin A new CDA I causative gene, C15ORF41, has been identified in
bridges linking two nuclei in more or less incompletely separated poly- three unrelated pedigrees of Middle Eastern and Southeast Asian ori-
chromatic erythroblasts with 0.6 to 2.8 strands per 100 erythroblasts gin by whole-genome sequencing. The cellular role of the restriction
20
(see Fig. 39–2A). Ultrastructural abnormalities consist of a spongy endonuclease encoded by the C15ORF41 gene is unknown. This protein
12
(“Swiss cheese”) appearance in the nucleus of up to 60 percent of early interacts with Asf1b, the paralogue of Asf1a, which may support the
and late polychromatic erythroblasts. Nuclei exhibit areas of electro- hypothesis that the primary defect in CDA I is confined to DNA repli-
lucency within electron-dense heterochromatin and contain nuclear cation and chromatin assembly.
membrane-lined cytoplasmic intrusions, occasionally with retained In the erythroid lineage during normal maturation, cell divi-
cytoplasmic organelles (see Fig. 39–2B). 9,11 sion ceases at the polychromatophilic erythroblast stage and cell size
Kaushansky_chapter 39_p0563-0570.indd 565 9/17/15 6:21 PM