Page 591 - Williams Hematology ( PDFDrive )
P. 591
566 Part VI: The Erythrocyte Chapter 39: The Congenital Dyserythropoietic Anemias 567
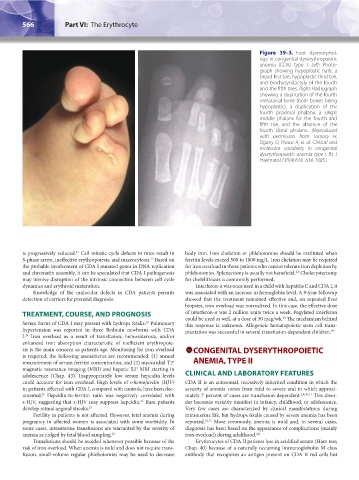
Figure 39–3. Foot dysmorphol-
ogy in congenital dyserythropoietic
anemia (CDA) type I. Left: Photo-
graph showing hypoplastic nails, a
broad first toe, hypoplastic third toe,
and brachysyndactyly of the fourth
and the fifth toes. Right: Radiograph
showing a duplication of the fourth
metatarsal bone (both bones being
hypoplastic), a duplication of the
fourth proximal phalanx, a single
middle phalanx for the fourth and
fifth toe, and the absence of the
fourth distal phalanx. (Reproduced
with permission from Tamary H,
Dgany O, Proust A, et al: Clinical and
molecular variability in congenital
dyserythropoietic anemia type I. Br J
Haematol 130(4):628–634, 2005.)
is progressively reduced. Cell mitotic cycle defects in mice result in body iron. Iron chelation or phlebotomies should be instituted when
21
S-phase arrest, ineffective erythropoiesis, and macrocytosis. Based on ferritin levels exceed 500 to 1000 mg/L. Iron chelation may be required
22
the probable involvement of CDA I mutated genes in DNA replication for iron overload in those patients who cannot tolerate iron depletion by
and chromatin assembly, it can be speculated that CDA I pathogenesis phlebotomies. Splenectomy is usually not beneficial. Cholecystectomy
2,9
may involve disruption of the intrinsic connection between cell cycle for cholelithiasis is commonly performed.
dynamics and erythroid maturation. Interferon-α was once used in a child with hepatitis C and CDA I; it
Knowledge of the molecular defects in CDA patients permits was associated with an increase in hemoglobin level. A 9-year followup
detection of carriers for prenatal diagnosis. showed that the treatment remained effective and, on repeated liver
biopsies, iron overload was normalized. In this case, the effective dose
TREATMENT, COURSE, AND PROGNOSIS of interferon-α was 2 million units twice a week. Pegylated interferon
could be used as well, at a dose of 30 mcg/wk. The mechanism behind
28
23
Severe forms of CDA I may present with hydrops fetalis. Pulmonary this response is unknown. Allogeneic hematopoietic stem cell trans-
hypertension was reported in three Bedouin newborns with CDA plantation was successful in several transfusion-dependent children. 29
I. Iron overload as a result of transfusion, hemosiderosis, and/or
24
enhanced iron absorption characteristic of inefficient erythropoie-
sis is the main concern as patients age. Monitoring for iron overload CONGENITAL DYSERYTHROPOIETIC
is required; the following assessments are recommended: (1) annual
*
measurement of serum ferritin concentration, and (2) myocardial T2 ANEMIA, TYPE II
magnetic resonance imaging (MRI) and hepatic R2 MRI starting in
*
adolescence (Chap. 43). Inappropriately low serum hepcidin levels CLINICAL AND LABORATORY FEATURES
could account for iron overload. High levels of s-hemojuvelin (HJV) CDA II is an autosomal, recessively inherited condition in which the
in patients affected with CDA I, compared with controls, have been doc- severity of anemia varies from mild to severe and in which approxi-
umented. Hepcidin-to-ferritin ratio was negatively correlated with mately 7 percent of cases are transfusion-dependent. 2,8,30,31 This disor-
25
s-HJV, suggesting that s-HJV may suppress hepcidin. Rare patients der becomes variably manifest in infancy, childhood, or adolescence.
26
develop retinal angioid streaks. 27 Very few cases are characterized by clinical manifestations during
Fertility in patients is not affected. However, fetal anemia during intrauterine life, but hydrops fetalis caused by severe anemia has been
pregnancy in affected women is associated with some morbidity. In reported. 32,33 More commonly, anemia is mild and, in several cases,
some cases, intrauterine transfusions are warranted by the severity of diagnosis has been based on the appearance of complications (mainly
anemia as judged by fetal blood sampling. 23 iron overload) during adulthood. 2,8
Transfusions should be avoided whenever possible because of the Erythrocytes of CDA II patients lyse in acidified serum (Ham test;
risk of iron overload. When anemia is mild and does not require trans- Chap. 40) because of a naturally occurring immunoglobulin M class
fusion, small-volume regular phlebotomies may be used to decrease antibody that recognizes an antigen present on CDA II red cells but
Kaushansky_chapter 39_p0563-0570.indd 566 9/17/15 6:21 PM