Page 721 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 721
694 Part SIX Systemic Immune Diseases
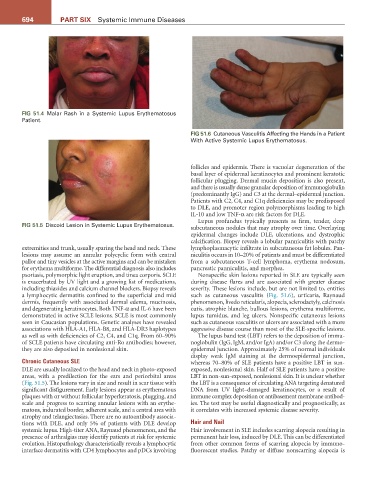
FIG 51.4 Malar Rash in a Systemic Lupus Erythematosus
Patient.
FIG 51.6 Cutaneous Vasculitis Affecting the Hands in a Patient
With Active Systemic Lupus Erythematosus.
follicles and epidermis. There is vacuolar degeneration of the
basal layer of epidermal keratinocytes and prominent keratotic
follicular plugging. Dermal mucin deposition is also present,
and there is usually dense granular deposition of immunoglobulin
(predominantly IgG) and C3 at the dermal–epidermal junction.
Patients with C2, C4, and C1q deficiencies may be predisposed
to DLE, and promoter region polymorphisms leading to high
IL-10 and low TNF-α are risk factors for DLE.
Lupus profundus typically presents as firm, tender, deep
FIG 51.5 Discoid Lesion in Systemic Lupus Erythematosus.
subcutaneous nodules that may atrophy over time. Overlaying
epidermal changes include DLE, ulcerations, and dystrophic
calcification. Biopsy reveals a lobular panniculitis with patchy
extremities and trunk, usually sparing the head and neck. These lymphoplasmacytic infiltrate in subcutaneous fat lobules. Pan-
lesions may assume an annular polycyclic form with central niculitis occurs in 10–20% of patients and must be differentiated
pallor and tiny vesicles at the active margins and can be mistaken from a subcutaneous T-cell lymphoma, erythema nodosum,
for erythema multiforme. The differential diagnosis also includes pancreatic panniculitis, and morphea.
psoriasis, polymorphic light eruption, and tinea corporis. SCLE Nonspecific skin lesions reported in SLE are typically seen
is exacerbated by UV light and a growing list of medications, during disease flares and are associated with greater disease
including thiazides and calcium channel blockers. Biopsy reveals severity. These lesions include, but are not limited to, entities
a lymphocytic dermatitis confined to the superficial and mid such as cutaneous vasculitis (Fig. 51.6), urticaria, Raynaud
dermis, frequently with associated dermal edema, mucinosis, phenomenon, livedo reticularis, alopecia, sclerodactyly, calcinosis
and degenerating keratinocytes. Both TNF-α and IL-6 have been cutis, atrophie blanche, bullous lesions, erythema multiforme,
demonstrated in active SCLE lesions. SCLE is most commonly lupus tumidus, and leg ulcers. Nonspecific cutaneous lesions
seen in Caucasian populations. Genetic analyses have revealed such as cutaneous vasculitis or ulcers are associated with a more
associations with HLA-A1, HLA-B8, and HLA-DR3 haplotypes aggressive disease course than most of the SLE-specific lesions.
as well as with deficiencies of C2, C4, and C1q. From 60–90% The lupus band test (LBT) refers to the deposition of immu-
of SCLE patients have circulating anti-Ro antibodies; however, noglobulin (IgG, IgM, and/or IgA) and/or C3 along the dermo-
they are also deposited in nonlesional skin. epidermal junction. Approximately 25% of normal individuals
display weak IgM staining at the dermoepidermal junction,
Chronic Cutaneous SLE whereas 70–80% of SLE patients have a positive LBT in sun-
DLE are usually localized to the head and neck in photo-exposed exposed, nonlesional skin. Half of SLE patients have a positive
areas, with a predilection for the ears and periorbital areas LBT in non-sun-exposed, nonlesional skin. It is unclear whether
(Fig. 51.5). The lesions vary in size and result in scar tissue with the LBT is a consequence of circulating ANA targeting denatured
significant disfigurement. Early lesions appear as erythematous DNA from UV light–damaged keratinocytes, or a result of
plaques with or without follicular hyperkeratosis, plugging, and immune complex deposition or antibasement membrane antibod-
scale and progress to scarring annular lesions with an erythe- ies. The test may be useful diagnostically and prognostically, as
matous, indurated border, adherent scale, and a central area with it correlates with increased systemic disease severity.
atrophy and telangiectasias. There are no autoantibody associa-
tions with DLE, and only 5% of patients with DLE develop Hair and Nail
systemic lupus. High-titer ANA, Raynaud phenomenon, and the Hair involvement in SLE includes scarring alopecia resulting in
presence of arthralgias may identify patients at risk for systemic permanent hair loss, induced by DLE. This can be differentiated
evolution. Histopathology characteristically reveals a lymphocytic from other common forms of scarring alopecia by immuno-
interface dermatitis with CD4 lymphocytes and pDCs involving fluorescent studies. Patchy or diffuse nonscarring alopecia is