Page 902 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 902
872 Part SEVEN Organ-Specific Inflammatory Disease
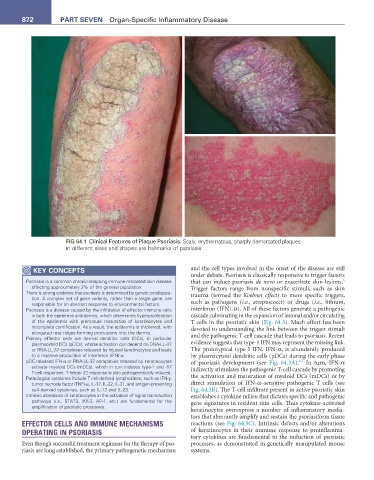
FIG 64.1 Clinical Features of Plaque Psoriasis. Scaly, erythematous, sharply demarcated plaques
in different sizes and shapes are hallmarks of psoriasis.
KEY CONCEPtS and the cell types involved in the onset of the disease are still
under debate. Psoriasis is classically responsive to trigger factors
Psoriasis is a common chronic-relapsing immune-mediated skin disease that can induce psoriasis de novo or exacerbate skin lesions.
6
affecting approximately 2% of the general population. Trigger factors range from nonspecific stimuli, such as skin
There is strong evidence that psoriasis is determined by genetic predisposi- trauma (termed the Koebner effect) to more specific triggers,
tion. A complex set of gene variants, rather than a single gene, are
responsible for an aberrant response to environmental factors. such as pathogens (i.e., streptococci) or drugs (i.e., lithium,
Psoriasis is a disease caused by the infiltration of effector immune cells interferon (IFN)-α). All of these factors generate a pathogenic
in both the epidermis and dermis, which determines hyperproliferation cascade culminating in the expansion of lesional and/or circulating
of the epidermis with premature maturation of keratinocytes and T cells in the psoriatic skin (Fig. 64.3). Much effort has been
incomplete cornification. As a result, the epidermis is thickened, with devoted to understanding the link between the trigger stimuli
elongated rete ridges forming protrusions into the dermis.
Primary effector cells are dermal dendritic cells (DCs), in particular and the pathogenic T-cell cascade that leads to psoriasis. Recent
plasmacytoid DCs (pDCs), whose activation can depend on DNA-LL-37 evidence suggests that type-1 IFN may represent the missing link.
or RNA-LL-37 complexes released by injured keratinocytes and leads The prototypical type I IFN, IFN-α, is abundantly produced
to a massive production of interferon (IFN)-α. by plasmacytoid dendritic cells (pDCs) during the early phase
pDC-released IFN-α or RNA-LL-37 complexes released by keratinocytes of psoriasis development (see Fig. 64.3A). In turn, IFN-α
6,7
activate myeloid DCs (mDCs), which in turn induces type-1 and -17 indirectly stimulates the pathogenic T-cell cascade by promoting
T-cell responses. T-helper 22 response is also pathogenetically induced.
Pathological cytokines include T-cell-derived lymphokines, such as IFN-γ, the activation and maturation of myeloid DCs (mDCs) or by
tumor necrosis factor (TNF)-α, IL-17, IL-22, IL-21, and antigen-presenting direct stimulation of IFN-α–sensitive pathogenic T cells (see
cell–derived cytokines, such as IL-12 and IL-23. Fig. 64.3B). The T-cell infiltrate present in active psoriatic skin
Intrinsic alterations of keratinocytes in the activation of signal transduction establishes a cytokine milieu that dictates specific and pathogenic
pathways (i.e., STAT3, IKK-2, AP-1, etc.) are fundamental for the gene signatures in resident skin cells. Thus cytokine-activated
amplification of psoriatic processes.
keratinocytes overexpress a number of inflammatory media-
tors that aberrantly amplify and sustain the psoriasiform tissue
EFFECTOR CELLS AND IMMUNE MECHANISMS reactions (see Fig. 64.3C). Intrinsic defects and/or alterations
OPERATING IN PSORIASIS of keratinocytes in their immune response to proinflamma-
tory cytokines are fundamental to the induction of psoriatic
Even though successful treatment regimens for the therapy of pso- processes, as demonstrated in genetically manipulated mouse
riasis are long established, the primary pathogenetic mechanism systems.