Page 23 - PRE-U STPM CHEMISTRY TERM 1
P. 23
ips
Tips
T
Exam
Exam
Chemistry Term 1 STPM
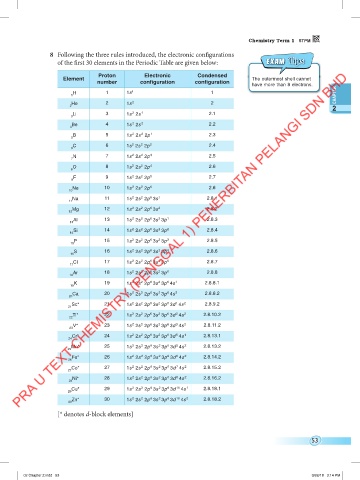
8 Following the three rules introduced, the electronic configurations
Tips
of the first 30 elements in the Periodic Table are given below: Exam T ips
Exam
Proton Electronic Condensed
Element The outermost shell cannot
number configuration configuration
have more than 8 electrons.
H 1 1s 1 1
1 CHAPTER
He 2 1s 2 2
2
2
2
Li 3 1s 2s 1 2.1
3
2
Be 4 1s 2s 2 2.2
4
2
2
B 5 1s 2s 2p 1 2.3
5
2
2
C 6 1s 2s 2p 2 2.4
6
2
2
N 7 1s 2s 2p 3 2.5
7
2
2
O 8 1s 2s 2p 4 2.6
8
2
2
F 9 1s 2s 2p 5 2.7
9
2
2
Ne 10 1s 2s 2p 6 2.8
10
2
2
6
Na 11 1s 2s 2p 3s 1 2.8.1
11
6
2
2
Mg 12 1s 2s 2p 3s 2 2.8.2
12
2
2
6
2
Al 13 1s 2s 2p 3s 3p 1 2.8.3
13
2
6
2
2
Si 14 1s 2s 2p 3s 3p 2 2.8.4
14
2
2
2
6
P 15 1s 2s 2p 3s 3p 3 2.8.5
15
2
2
6
2
S 16 1s 2s 2p 3s 3p 4 2.8.6
16
2
2
2
6
Cl 17 1s 2s 2p 3s 3p 5 2.8.7
17
2
2
2
6
Ar 18 1s 2s 2p 3s 3p 6 2.8.8
18
6
2
2
6
2
K 19 1s 2s 2p 3s 3p 4s 1 2.8.8.1
19
2
6
2
2
6
Ca 20 1s 2s 2p 3s 3p 4s 2 2.8.8.2
20
2
1
2
6
6
2
Sc* 21 1s 2s 2p 3s 3p 3d 4s 2 2.8.9.2
21
6
2
6
2
2
2
Ti* 22 1s 2s 2p 3s 3p 3d 4s 2 2.8.10.2
22
6
2
6
2
2
3
V* 23 1s 2s 2p 3s 3p 3d 4s 2 2.8.11.2
23
6
2
5
2
6
2
Cr* 24 1s 2s 2p 3s 3p 3d 4s 1 2.8.13.1
24
6
5
2
6
2
2
Mn* 25 1s 2s 2p 3s 3p 3d 4s 2 2.8.13.2
25
2
2
6
6
6
2
Fe* 26 1s 2s 2p 3s 3p 3d 4s 2 2.8.14.2
26
2
6
7
6
2
2
Co* 27 1s 2s 2p 3s 3p 3d 4s 2 2.8.15.2
27
6
2
6
2
8
2
Ni* 28 1s 2s 2p 3s 3p 3d 4s 2 2.8.16.2
28
6
6
2
2
2
Cu* 29 1s 2s 2p 3s 3p 3d 10 4s 1 2.8.18.1
29
2
2
6
6
2
Zn* 30 1s 2s 2p 3s 3p 3d 10 4s 2 2.8.18.2
30
[* denotes d-block elements]
53
02 Chapter 2.indd 53 3/26/18 3:14 PM