Page 56 - PRE-U STPM CHEMISTRY TERM 1
P. 56
Chemistry Term 1 STPM
4 (a) A mixture with a constant boiling point. Its 6 (a) (i) (ii)
composition remains constant on distillation.
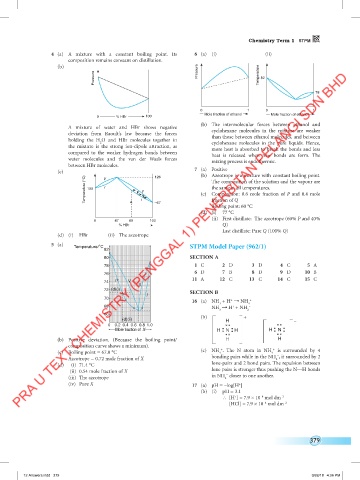
(b) Pressure
Pressure Temperature 82
78
0 1 0 1
Mole fraction of ethanol Mole fraction of ethanol
0 % HBr 100
(b) The intermolecular forces between ethanol and
A mixture of water and HBr shows negative cyclohexane molecules in the mixture are weaker
deviation from Raoult’s law because the forces than those between ethanol molecules, and between
holding the H O and HBr molecules together in
2 cyclohexane molecules in the pure liquids. Hence,
the mixture is the strong ion-dipole attraction, as more heat is absorbed to break the bonds and less
compared to the weaker hydrogen bonds between heat is released when new bonds are form. The
water molecules and the van der Waals forces mixing process is endothermic.
between HBr molecules.
(c) 126 7 (a) Positive
(b) Azeotrope is a mixture with constant boiling point.
Temperature (°C) 100 l V (c) Composition: 0.6 mole fraction of P and 0.4 mole
V The composition of the solution and the vapour are
the same at all temperatures.
l
–67
Boiling point: 60 °C
fraction of Q
(d) (i) 77 °C
(ii) First distillate: The azeotrope (60% P and 40%
0 47 60 100
% HBr Q)
Last distillate: Pure Q (100% Q)
(d) (i) HBr (ii) The azeotrope
5 (a)
Temperature/°C STPM Model Paper (962/1)
82
80 SECTION A
78 1 C 2 D 3 D 4 C 5 A
6 D 7 B 8 D 9 D 10 B
76
11 A 12 C 13 C 14 C 15 C
74 l V
V
72 (d)(i) SECTION B
l
70
16 (a) NH + H : NH 4 +
+
3
+
68 NH : H + NH –
3 2
66 (b) + +
(d)(ii) H H – –
0 0.2 0.4 0.6 0.8 1.0
Mole fraction of X H N H H N H H N H N
(b) Positive deviation. (Because the boiling point/ H H H H
composition curve shows a minimum).
+
+
(c) Boiling point = 67.0 °C (c) NH . The N atom in NH is surrounded by 4
4
4
–
Azeotrope = 0.72 mole fraction of X bonding pairs while in the NH , it surrounded by 2
2
(d) (i) 71.4 °C lone-pairs and 2 bond pairs. The repulsion between
(ii) 0.54 mole fraction of X lone pairs is stronger thus pushing the N9H bonds
–
(iii) The azeotrope in NH closer to one another.
2
(iv) Pure X 17 (a) pH = –log[H ]
+
(b) (i) pH = 3.1
∴ [H ] = 7.9 × 10 mol dm –3
–4
+
[HCl] = 7.9 × 10 mol dm –2
–4
379
12 Answers.indd 379 3/26/18 4:06 PM