Page 28 - Hybrid PBD 2022 Tg 5 - Kimia
P. 28
Kimia Tingkatan 5 Bab 3
BAB
3 Termokimia
Thermochemistry
Penerbitan Pelangi Sdn Bhd. All Rights Reserved
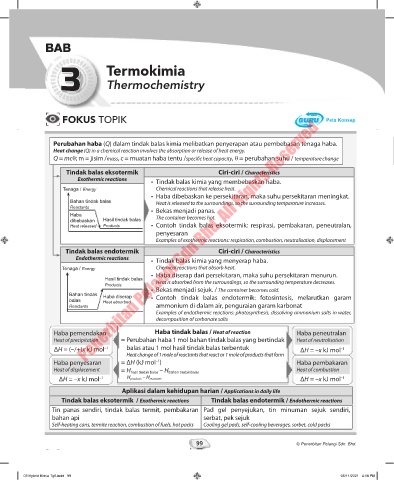
FOKUS TOPIK Peta Konsep
Perubahan haba (Q) dalam tindak balas kimia melibatkan penyerapan atau pembebasan tenaga haba.
Heat change (Q) in a chemical reaction involves the absorption or release of heat energy.
Q = mcθ; m = jisim /mass, c = muatan haba tentu /specific heat capacity, θ = perubahan suhu / temperature change
Tindak balas eksotermik Ciri-ciri / Characteristics
Exothermic reactions • Tindak balas kimia yang membebaskan haba.
Tenaga / Energy Chemical reactions that release heat.
• Haba dibebaskan ke persekitaran, maka suhu persekitaran meningkat.
Bahan tindak balas Heat is released to the surroundings, so the surrounding temperature increases.
Reactants • Bekas menjadi panas.
Haba The container becomes hot.
dibebaskan Hasil tindak balas
Heat released Products • Contoh tindak balas eksotermik: respirasi, pembakaran, peneutralan,
penyesaran
Examples of exothermic reactions: respiration, combustion, neutralisation, displacement
Tindak balas endotermik Ciri-ciri / Characteristics
Endothermic reactions • Tindak balas kimia yang menyerap haba.
Tenaga / Energy Chemical reactions that absorb heat.
• Haba diserap dari persekitaran, maka suhu persekitaran menurun.
Hasil tindak balas Heat is absorbed from the surroundings, so the surrounding temperature decreases.
Products
• Bekas menjadi sejuk. / The container becomes cold.
Bahan tindak Haba diserap • Contoh tindak balas endotermik: fotosintesis, melarutkan garam
balas Heat absorbed
Reactants ammonium di dalam air, penguraian garam karbonat
Examples of endothermic reactions: photosynthesis, dissolving ammonium salts in water,
decomposition of carbonate salts
Haba pemendakan Haba tindak balas / Heat of reaction Haba peneutralan
Heat of precipitation = Perubahan haba 1 mol bahan tindak balas yang bertindak Heat of neutralisation
ΔH = (–/+)x kJ mol –1 balas atau 1 mol hasil tindak balas terbentuk ΔH = –x kJ mol –1
Heat change of 1 mole of reactants that react or 1 mole of products that form
Haba penyesaran = ΔH (kJ mol ) Haba pembakaran
–1
Heat of displacement = H hasil tindak balas – H bahan tindak balas Heat of combustion
ΔH = –x kJ mol –1 H products – H reactants ΔH = –x kJ mol –1
Aplikasi dalam kehidupan harian / Applications in daily life
Tindak balas eksotermik / Exothermic reactions Tindak balas endotermik / Endothermic reactions
Tin panas sendiri, tindak balas termit, pembakaran Pad gel penyejukan, tin minuman sejuk sendiri,
bahan api serbat, pek sejuk
Self-heating cans, termite reaction, combustion of fuels, hot packs Cooling gel pads, self-cooling beverages, sorbet, cold packs
99 © Penerbitan Pelangi Sdn. Bhd.
03 Hybrid Kimia Tg5.indd 99 05/11/2021 4:18 PM