Page 29 - Focus SPM KSSM F4 2020 - Chemistry
P. 29
Chemistry For m 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
Chemistry Form 4 Chapter 3 The Mole Concept, Chemical Formula and Equation
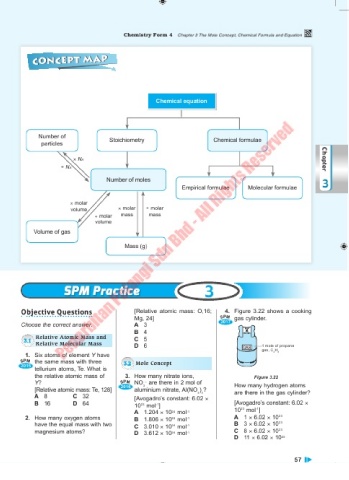
CONCEPT MAP
CONCEPT MAP
Chemical equation
Number of
particles Stoichiometry Chemical formulae
× NA Chapter
÷ NA
Number of moles 3
Empirical formulae Molecular formulae
× molar
volume × molar ÷ molar
÷ molar mass mass
volume
Volume of gas
Mass (g)
SPM Practice 3
Objective Questions [Relative atomic mass: O,16; 4. Figure 3.22 shows a cooking
Mg, 24] SPM gas cylinder.
Choose the correct answer. A 3 2017
B 4
Relative Atomic Mass and C 5
3.1
Relative Molecular Mass D 6 1 mole of propane
gas, C H
1. Six atoms of element Y have 3 8
SPM the same mass with three 3.2 Mole Concept
2015
tellurium atoms, Te. What is
the relative atomic mass of 3. How many nitrate ions, Figure 3.22
Y? SPM NO are there in 2 mol of How many hydrogen atoms
–
3
[Relative atomic mass: Te, 128] 2016 aluminium nitrate, Al(NO ) ? are there in the gas cylinder?
3 3
A 8 C 32 [Avogadro’s constant: 6.02 ×
B 16 D 64 10 mol ] [Avogadro’s constant: 6.02 ×
-1
23
-1
23
24
A 1.204 × 10 mol -1 10 mol ]
23
2. How many oxygen atoms B 1.806 × 10 mol -1 A 1 × 6.02 × 10
24
23
have the equal mass with two C 3.010 × 10 mol -1 B 3 × 6.02 × 10
24
23
magnesium atoms? D 3.612 × 10 mol -1 C 8 × 6.02 × 10
24
D 11 × 6.02 × 10
23
57
57
03 SPM CHEMISTRY F4.indd 57 27/02/2020 11:23 AM