Page 2 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 2
Chemistry Terminologies
Chapter 1 : Matter Chemistry Unit KMNS
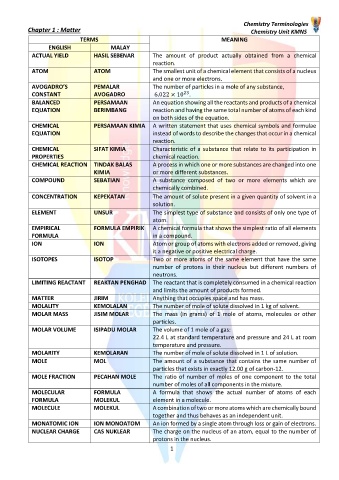
TERMS MEANING
ENGLISH MALAY
ACTUAL YIELD HASIL SEBENAR The amount of product actually obtained from a chemical
reaction.
ATOM ATOM The smallest unit of a chemical element that consists of a nucleus
and one or more electrons.
AVOGADRO’S PEMALAR The number of particles in a mole of any substance,
23
CONSTANT AVOGADRO 6.022 × 10 .
BALANCED PERSAMAAN An equation showing all the reactants and products of a chemical
EQUATION BERIMBANG reaction and having the same total number of atoms of each kind
on both sides of the equation.
CHEMICAL PERSAMAAN KIMIA A written statement that uses chemical symbols and formulae
EQUATION instead of words to describe the changes that occur in a chemical
reaction.
CHEMICAL SIFAT KIMIA Characteristic of a substance that relate to its participation in
PROPERTIES chemical reaction.
CHEMICAL REACTION TINDAK BALAS A process in which one or more substances are changed into one
KIMIA or more different substances.
COMPOUND SEBATIAN A substance composed of two or more elements which are
chemically combined.
CONCENTRATION KEPEKATAN The amount of solute present in a given quantity of solvent in a
solution.
ELEMENT UNSUR The simplest type of substance and consists of only one type of
atom.
EMPIRICAL FORMULA EMPIRIK A chemical formula that shows the simplest ratio of all elements
FORMULA in a compound.
ION ION Atom or group of atoms with electrons added or removed, giving
it a negative or positive electrical charge.
ISOTOPES ISOTOP Two or more atoms of the same element that have the same
number of protons in their nucleus but different numbers of
neutrons.
LIMITING REACTANT REAKTAN PENGHAD The reactant that is completely consumed in a chemical reaction
and limits the amount of products formed.
MATTER JIRIM Anything that occupies space and has mass.
MOLALITY KEMOLALAN The number of mole of solute dissolved in 1 kg of solvent.
MOLAR MASS JISIM MOLAR The mass (in grams) of 1 mole of atoms, molecules or other
particles.
MOLAR VOLUME ISIPADU MOLAR The volume of 1 mole of a gas:
22.4 L at standard temperature and pressure and 24 L at room
temperature and pressure.
MOLARITY KEMOLARAN The number of mole of solute dissolved in 1 L of solution.
MOLE MOL The amount of a substance that contains the same number of
particles that exists in exactly 12.00 g of carbon-12.
MOLE FRACTION PECAHAN MOLE The ratio of number of moles of one component to the total
number of moles of all components in the mixture.
MOLECULAR FORMULA A formula that shows the actual number of atoms of each
FORMULA MOLEKUL element in a molecule.
MOLECULE MOLEKUL A combination of two or more atoms which are chemically bound
together and thus behaves as an independent unit.
MONATOMIC ION ION MONOATOM An ion formed by a single atom through loss or gain of electrons.
NUCLEAR CHARGE CAS NUKLEAR The charge on the nucleus of an atom, equal to the number of
protons in the nucleus.
1