Page 4 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 4
Chemistry Terminologies
Chapter 2 : Atomic Structure
Chemistry Unit KMNS
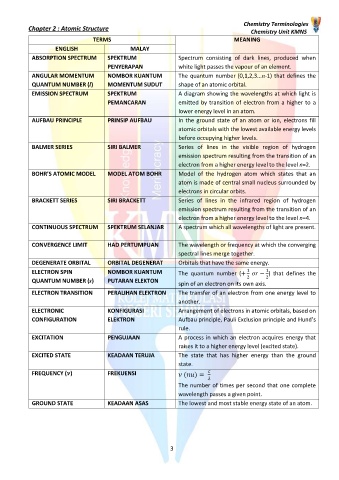
TERMS MEANING
ENGLISH MALAY
ABSORPTION SPECTRUM SPEKTRUM Spectrum consisting of dark lines, produced when
PENYERAPAN white light passes the vapour of an element.
ANGULAR MOMENTUM NOMBOR KUANTUM The quantum number (0,1,2,3...n-1) that defines the
QUANTUM NUMBER (l) MOMENTUM SUDUT shape of an atomic orbital.
EMISSION SPECTRUM SPEKTRUM A diagram showing the wavelengths at which light is
PEMANCARAN emitted by transition of electron from a higher to a
lower energy level in an atom.
AUFBAU PRINCIPLE PRINSIP AUFBAU In the ground state of an atom or ion, electrons fill
atomic orbitals with the lowest available energy levels
before occupying higher levels.
BALMER SERIES SIRI BALMER Series of lines in the visible region of hydrogen
emission spectrum resulting from the transition of an
electron from a higher energy level to the level n=2.
BOHR’S ATOMIC MODEL MODEL ATOM BOHR Model of the hydrogen atom which states that an
atom is made of central small nucleus surrounded by
electrons in circular orbits.
BRACKETT SERIES SIRI BRACKETT Series of lines in the infrared region of hydrogen
emission spectrum resulting from the transition of an
electron from a higher energy level to the level n=4.
CONTINUOUS SPECTRUM SPEKTRUM SELANJAR A spectrum which all wavelengths of light are present.
CONVERGENCE LIMIT HAD PERTUMPUAN The wavelength or frequency at which the converging
spectral lines merge together.
DEGENERATE ORBITAL ORBITAL DEGENERAT Orbitals that have the same energy.
1
1
ELECTRON SPIN NOMBOR KUANTUM The quantum number (+ − ) that defines the
2 2
QUANTUM NUMBER (s) PUTARAN ELEKTON
spin of an electron on its own axis.
ELECTRON TRANSITION PERALIHAN ELEKTRON The transfer of an electron from one energy level to
another.
ELECTRONIC KONFIGURASI Arrangement of electrons in atomic orbitals, based on
CONFIGURATION ELEKTRON Aufbau principle, Pauli Exclusion principle and Hund’s
rule.
EXCITATION PENGUJAAN A process in which an electron acquires energy that
raises it to a higher energy level (excited state).
EXCITED STATE KEADAAN TERUJA The state that has higher energy than the ground
state.
FREQUENCY ( ) FREKUENSI ( ) =
The number of times per second that one complete
wavelength passes a given point.
GROUND STATE KEADAAN ASAS The lowest and most stable energy state of an atom.
3