Page 5 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 5
Chemistry Terminologies
Chemistry Unit KMNS
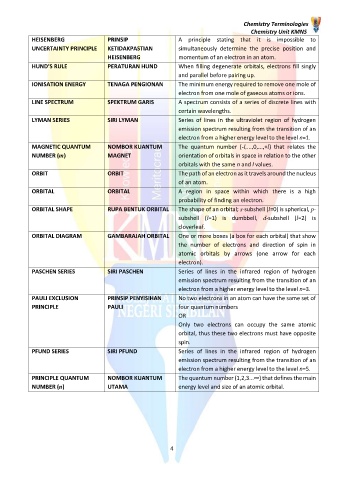
HEISENBERG PRINSIP A principle stating that it is impossible to
UNCERTAINTY PRINCIPLE KETIDAKPASTIAN simultaneously determine the precise position and
HEISENBERG momentum of an electron in an atom.
HUND’S RULE PERATURAN HUND When filling degenerate orbitals, electrons fill singly
and parallel before pairing up.
IONISATION ENERGY TENAGA PENGIONAN The minimum energy required to remove one mole of
electron from one mole of gaseous atoms or ions.
LINE SPECTRUM SPEKTRUM GARIS A spectrum consists of a series of discrete lines with
certain wavelengths.
LYMAN SERIES SIRI LYMAN Series of lines in the ultraviolet region of hydrogen
emission spectrum resulting from the transition of an
electron from a higher energy level to the level n=1.
MAGNETIC QUANTUM NOMBOR KUANTUM The quantum number (-l,...,0,...,+l) that relates the
NUMBER (m) MAGNET orientation of orbitals in space in relation to the other
orbitals with the same n and l values.
ORBIT ORBIT The path of an electron as it travels around the nucleus
of an atom.
ORBITAL ORBITAL A region in space within which there is a high
probability of finding an electron.
ORBITAL SHAPE RUPA BENTUK ORBITAL The shape of an orbital; s-subshell (l=0) is spherical, p-
subshell (l=1) is dumbbell, d-subshell (l=2) is
cloverleaf.
ORBITAL DIAGRAM GAMBARAJAH ORBITAL One or more boxes (a box for each orbital) that show
the number of electrons and direction of spin in
atomic orbitals by arrows (one arrow for each
electron).
PASCHEN SERIES SIRI PASCHEN Series of lines in the infrared region of hydrogen
emission spectrum resulting from the transition of an
electron from a higher energy level to the level n=3.
PAULI EXCLUSION PRINSIP PENYISIHAN No two electrons in an atom can have the same set of
PRINCIPLE PAULI four quantum numbers
OR
Only two electrons can occupy the same atomic
orbital, thus these two electrons must have opposite
spin.
PFUND SERIES SIRI PFUND Series of lines in the infrared region of hydrogen
emission spectrum resulting from the transition of an
electron from a higher energy level to the level n=5.
PRINCIPLE QUANTUM NOMBOR KUANTUM The quantum number (1,2,3...∞) that defines the main
NUMBER (n) UTAMA energy level and size of an atomic orbital.
4