Page 112 - Nilam_Publication_module_Chemistry_Form.pdf
P. 112
MODULE • Chemistry Form 4
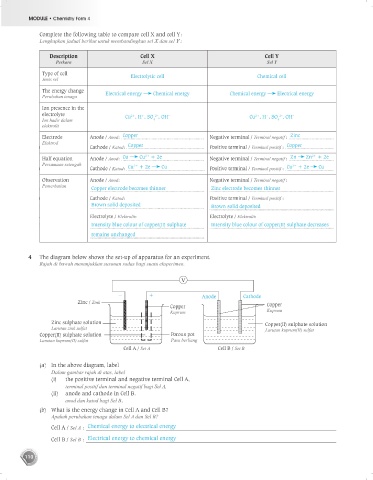
Complete the following table to compare cell X and cell Y :
Lengkapkan jadual berikut untuk membandingkan sel X dan sel Y :
Description Cell X Cell Y
Perkara Sel X Sel Y
Type of cell Electrolytic cell Chemical cell
Jenis sel
The energy change Electrical energy Chemical energy Chemical energy Electrical energy
Perubahan tenaga
Ion presence in the
electrolyte Cu , H , SO , OH – Cu , H , SO , OH –
2+
2–
+
2+
+
2–
Ion hadir dalam 4 4
elektrolit
Electrode Anode / Anod: Copper Negative terminal / Terminal negatif : Zinc
Elektrod Copper Copper
Cathode / Katod: Positive terminal / Terminal positif :
2+
2+
Half equation Anode / Anod: Cu Cu + 2e Negative terminal / Terminal negatif : Zn Zn + 2e
Persamaan setengah Cu + 2e Cu Cu + 2e Cu
2+
2+
Cathode / Katod: Positive terminal / Terminal positif :
Observation Anode / Anod: Negative terminal / Terminal negatif :
Pemerhatian Copper electrode becomes thinner Zinc electrode becomes thinner
Cathode / Katod: Positive terminal / Terminal positif :
Brown solid deposited Brown solid deposited
Electrolyte / Elektrolit: Electrolyte / Elektrolit:
Intensity blue colour of copper(II) sulphate Intensity blue colour of copper(II) sulphate decreases
remains unchanged
4 The diagram below shows the set-up of apparatus for an experiment.
Rajah di bawah menunjukkan susunan radas bagi suatu eksperimen.
V
– + Anode Cathode
Zinc / Zink Copper
Copper
Kuprum Kuprum
Zinc sulphate solution Copper(II) sulphate solution
Larutan zink sulfat Larutan kuprum(II) sulfat
Copper(II) sulphate solution Porous pot
Larutan kuprum(II) sulfat Pasu berliang
Cell A / Set A Cell B / Set B
(a) In the above diagram, label
Dalam gambar rajah di atas, label
(i) the positive terminal and negative terminal Cell A,
terminal positif dan terminal negatif bagi Sel A,
(ii) anode and cathode in Cell B.
anod dan katod bagi Sel B.
(b) What is the energy change in Cell A and Cell B?
Apakah perubahan tenaga dalam Sel A dan Sel B?
Cell A / Sel A : Chemical energy to electrical energy
Cell B / Sel B : Electrical energy to chemical energy
110
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 110 12/9/2011 5:56:32 PM