Page 12 - Nilam_Publication_module_Chemistry_Form.pdf
P. 12
MODULE • Chemistry Form 4
(a) (i) What is meant by ‘melting point’?
Apakah yang dimaksudkan dengan ‘takat lebur’?
The constant temperature at which a solid charges to a liquid at particular pressure
(ii) What is meant by ‘boiling point’?
Apakah yang dimaksudkan dengan ‘takat didih’?
The constant temperature at which a liquid changes to a gas at particular pressure
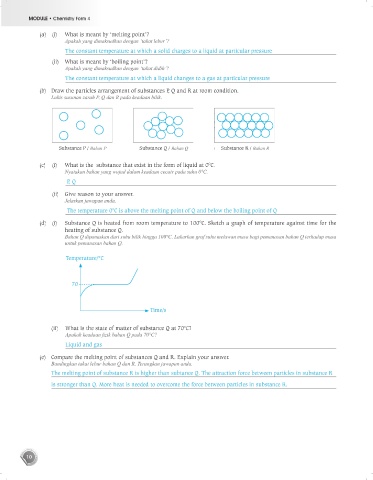
(b) Draw the particles arrangement of substances P, Q and R at room condition.
Lukis susunan zarah P, Q dan R pada keadaan bilik.
Substance P / Bahan P Substance Q / Bahan Q Substance R / Bahan R
(c) (i) What is the substance that exist in the form of liquid at 0°C.
Nyatakan bahan yang wujud dalam keadaan cecair pada suhu 0°C.
P, Q
(ii) Give reason to your answer.
Jelaskan jawapan anda.
The temperature 0°C is above the melting point of Q and below the boiling point of Q
(d) (i) Substance Q is heated from room temperature to 100°C. Sketch a graph of temperature against time for the
heating of substance Q.
Bahan Q dipanaskan dari suhu bilik hingga 100°C. Lakarkan graf suhu melawan masa bagi pemanasan bahan Q terhadap masa
untuk pemanasan bahan Q.
Temperature/°C
70
Time/s
(ii) What is the state of matter of substance Q at 70°C?
Apakah keadaan fizik bahan Q pada 70°C?
Liquid and gas
(e) Compare the melting point of substances Q and R. Explain your answer.
Bandingkan takat lebur bahan Q dan R. Terangkan jawapan anda.
The melting point of substance R is higher than subtance Q. The attraction force between particles in substance R
is stronger than Q. More heat is needed to overcome the force between particles in substance R.
10
Nilam Publication Sdn. Bhd.
01-Chem F4 (3p).indd 10 12/9/2011 5:59:30 PM