Page 142 - Nilam_Publication_module_Chemistry_Form.pdf
P. 142
MODULE • Chemistry Form 4
PREPARATION OF SALT / PENYEDIAAN GARAM
1 A salt is a compound formed when the hydrogen ion in an acid is replaced with metal ion or ammonium ion. Example:
Sodium chloride, copper(II) sulphate, potassium nitrate and ammonium sulphate.
Garam ialah sebatian ion yang terhasil apabila ion hidrogen daripada asid diganti oleh ion logam termasuk ion ammonium.
Contoh: natrium klorida, kuprum(II) sulfat, kalium nitrat dan ammonium sulfat.
2 Write the formulae of the salts in the table below by replacing hydrogen ion in sulphuric acid, hydrochloric acid, nitric
acid and carbonic acid with metal ions or ammonium ion.
Tuliskan formula kimia garam berikut dengan menggantikan ion hidrogen dalam asid sulfurik, asid hidroklorik, asid nitrik dan asid
karbonik dengan ion logam atau ion ammonium:
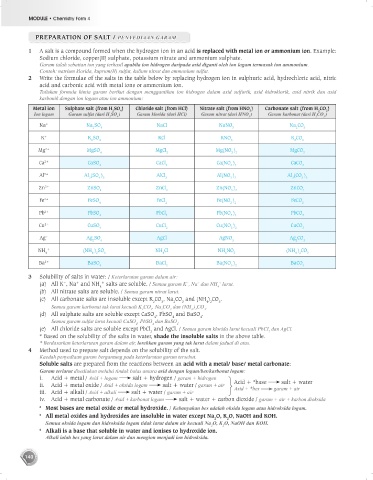
Metal ion Sulphate salt (from H SO ) Chloride salt (from HCl) Nitrate salt (from HNO ) Carbonate salt (from H CO )
3
3
2
2
4
Ion logam Garam sulfat (dari H SO ) Garam klorida (dari HCl) Garam nitrat (dari HNO ) Garam karbonat (dari H CO )
2 4 3 2 3
Na + Na SO NaCl NaNO Na CO
2 4 3 2 3
K + K SO KCl KNO K CO
2 4 3 2 3
Mg 2+ MgSO MgCl Mg(NO ) MgCO
4 2 3 2 3
Ca 2+ CaSO CaCl Ca(NO ) CaCO
4 2 3 2 3
Al 3+ Al (SO ) AlCl Al(NO ) Al (CO )
2 4 3 3 3 3 2 3 3
Zn 2+ ZnSO ZnCl Zn(NO ) ZnCO
4 2 3 2 3
Fe 2+ FeSO FeCl Fe(NO ) FeCO
4 2 3 2 3
Pb 2+ PbSO PbCl Pb(NO ) PbCO
4 2 3 2 3
Cu 2+ CuSO CuCl Cu(NO ) CuCO
4 2 3 2 3
Ag + Ag SO AgCl AgNO Ag CO
2 4 3 2 3
NH + (NH ) SO NH Cl NH NO (NH ) CO
4 4 2 4 4 4 3 4 2 3
Ba 2+ BaSO BaCl Ba(NO ) BaCO
4 2 3 2 3
3 Solubility of salts in water: / Keterlarutan garam dalam air:
+
+
+
+
(a) All K , Na and NH salts are soluble. / Semua garam K , Na dan NH larut.
+
+
4 4
(b) All nitrate salts are soluble. / Semua garam nitrat larut.
(c) All carbonate salts are insoluble except K CO , Na CO and (NH ) CO .
3
2
4 2
3
2
3
Semua garam karbonat tak larut kecuali K CO , Na CO dan (NH ) CO .
2
3
3
2
4 2
3
(d) All sulphate salts are soluble except CaSO , PbSO and BaSO .
4
4
4
Semua garam sulfat larut kecuali CaSO , PbSO dan BaSO .
4 4 4
(e) All chloride salts are soluble except PbCl and AgCl. / Semua garam klorida larut kecuali PbCl dan AgCl.
2
2
* Based on the solubility of the salts in water, shade the insoluble salts in the above table.
* Berdasarkan keterlarutan garam dalam air, lorekkan garam yang tak larut dalam jadual di atas.
4 Method used to prepare salt depends on the solubility of the salt.
Kaedah penyediaan garam bergantung pada keterlarutan garam tersebut.
Soluble salts are prepared from the reactions between an acid with a metal/ base/ metal carbonate:
Garam terlarut disediakan melalui tindak balas antara asid dengan logam/bes/karbonat logam:
i. Acid + metal / Asid + logam salt + hydrogen / garam + hidrogen
salt + water
ii. Acid + metal oxide / Asid + oksida logam salt + water / garam + air Acid + *base garam + air
Asid + *bes
iii. Acid + alkali / Asid + alkali salt + water / garam + air
iv. Acid + metal carbonate / Asid + karbonat logam salt + water + carbon dioxide / garam + air + karbon dioksida
* Most bases are metal oxide or metal hydroxide. / Kebanyakan bes adalah oksida logam atau hidroksida logam.
* All metal oxides and hydroxides are insoluble in water except Na O, K O, NaOH and KOH.
2 2
Semua oksida logam dan hidroksida logam tidak larut dalam air kecuali Na O, K O, NaOH dan KOH.
2 2
* Alkali is a base that soluble in water and ionises to hydroxide ion.
Alkali ialah bes yang larut dalam air dan mengion menjadi ion hidroksida.
140
Nilam Publication Sdn. Bhd.
07-Chem F4 (3p).indd 140 12/9/2011 5:55:19 PM