Page 145 - Nilam_Publication_module_Chemistry_Form.pdf
P. 145
Chemistry Form 4 • MODULE
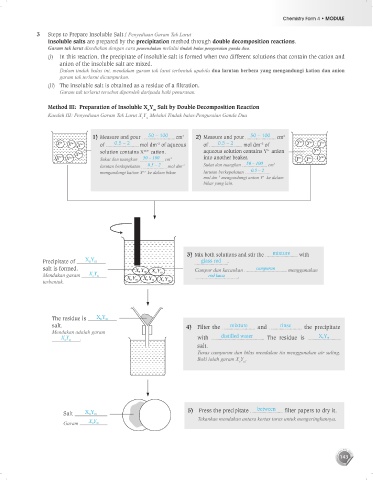
3 Steps to Prepare Insoluble Salt / Penyediaan Garam Tak Larut
Insoluble salts are prepared by the precipitation method through double decomposition reactions.
Garam tak larut disediakan dengan cara pemendakan melalui tindak balas penguraian ganda dua.
(i) In this reaction, the precipitate of insoluble salt is formed when two different solutions that contain the cation and
anion of the insoluble salt are mixed.
Dalam tindak balas ini, mendakan garam tak larut terbentuk apabila dua larutan berbeza yang mengandungi kation dan anion
garam tak terlarut dicampurkan.
(ii) The insoluble salt is obtained as a residue of a filtration.
Garam tak terlarut tersebut diperoleh daripada baki penurasan.
Method III: Preparation of Insoluble X Y Salt by Double Decomposition Reaction
n m
Kaedah III: Penyediaan Garam Tak Larut X Y Melalui Tindak balas Penguraian Ganda Dua
n m
1) Measure and pour 50 – 100 cm 2) Measure and pour 50 – 100 cm 3
3
of 0.5 – 2 mol dm of aqueous of 0.5 – 2 mol dm of
–3
–3
n–
solution contains X m+ cation. aqueous solution contains Y anion
Sukat dan tuangkan 50 – 100 cm 3 into another beaker.
larutan berkepekatan 0.5 – 2 mol dm –3 Sukat dan tuangkan 50 – 100 cm 3
mengandungi kation X ke dalam bikar. larutan berkepekatan 0.5 – 2
m+
n–
mol dm mengandungi anion Y ke dalam
–3
bikar yang lain.
3) Mix both solutions and stir the mixture with
Precipitate of X n Y m glass rod .
salt is formed. Campur dan kacaukan campuran menggunakan
Mendakan garam X Y rod kaca .
n m
terbentuk.
The residue is X n Y m
salt. 4) Filter the mixture and rinse the precipitate
Mendakan adalah garam
X Y . with distilled water . The residue is X n Y m
n m
salt.
Turas campuran dan bilas mendakan itu menggunakan air suling.
Baki ialah garam X Y .
n m
Salt X n Y m 5) Press the precipitate between filter papers to dry it.
Tekankan mendakan antara kertas turas untuk mengeringkannya.
Garam X n Y m
143
Nilam Publication Sdn. Bhd.
07-Chem F4 (3p).indd 143 12/9/2011 5:55:21 PM