Page 146 - Nilam_Publication_module_Chemistry_Form.pdf
P. 146
MODULE • Chemistry Form 4
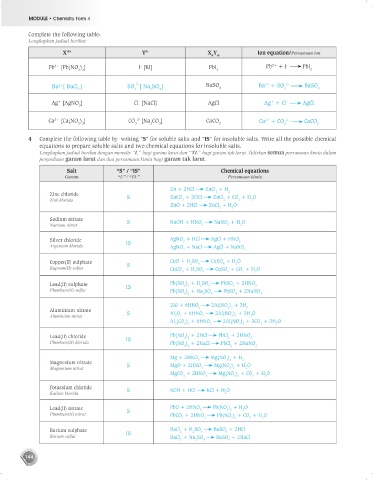
Complete the following table:
Lengkapkan jadual berikut:
X m+ Y n– X Y m Ion equation/Persamaan ion
n
–
2+
Pb [Pb(NO ) ] I [KI] PbI Pb + I PbI
2+
–
3 2 2 2
2–
2+
2–
Ba [ BaCl ] SO [ Na SO ] BaSO 4 Ba + SO BaSO 4
2+
4
2
4
2
4
Ag [AgNO ] Cl [NaCl] AgCl Ag + Cl – AgCl
+
+
–
3
Ca [Ca(NO ) ] CO 2– [Na CO ] CaCO Ca + CO CaCO
2+
2–
2+
3 2 3 2 3 3 3 3
4 Complete the following table by writing “S” for soluble salts and “IS” for insoluble salts. Write all the possible chemical
equations to prepare soluble salts and two chemical equations for insoluble salts.
Lengkapkan jadual berikut dengan menulis “L” bagi garam larut dan “TL” bagi garam tak larut. Tuliskan semua persamaan kimia dalam
penyediaan garam larut dan dua persamaan kimia bagi garam tak larut.
Salt “S” / “IS” Chemical equations
Garam “L” / “TL” Persamaan kimia
Zn + 2HCl ZnCl + H
2
2
Zinc chloride S ZnCO + 2HCl ZnCl + CO + H O
Zink klorida 3 2 2 2
ZnO + 2HCl ZnCl + H O
2 2
Sodium nitrate S NaOH + HNO NaNO + H O
Natrium nitrat 3 3 2
Silver chloride IS AgNO + HCl AgCl + HNO 3
3
Argentum klorida AgNO + NaCl AgCl + NaNO
3 3
Copper(II) sulphate S CuO + H SO CuSO + H O
4
2
2
4
Kuprum(II) sulfat CuCO + H SO CuSO + CO + H O
3 2 4 4 2 2
Lead(II) sulphate IS Pb(NO ) + H SO PbSO + 2HNO 3
3 2
2
4
4
Plumbum(II) sulfat Pb(NO ) + Na SO PbSO + 2NaNO
3 2 2 4 4 3
2Al + 6HNO 2Al(NO ) + 3H
3
3 3
2
Aluminium nitrate S Al O + 6HNO 2Al(NO ) + 3H O
Aluminium nitrat 2 3 3 3 3 2
Al (CO ) + 6HNO 2Al(NO ) + 3CO + 3H O
2 3 3 3 3 3 2 2
Lead(II) chloride IS Pb(NO ) + 2HCl PbCl + 2HNO 3
3 2
2
Plumbum(II) klorida Pb(NO ) + 2NaCl PbCl + 2NaNO
3 2 2 3
Mg + 2HNO Mg(NO ) + H 2
3
3 2
Magnesium nitrate S MgO + 2HNO Mg(NO ) + H O
Magnesium nitrat 3 3 2 2
MgCO + 2HNO Mg(NO ) + CO + H O
3 3 3 2 2 2
Potassium chloride S KOH + HCl KCl + H O
Kalium klorida 2
Lead(II) nitrate S PbO + 2HNO Pb(NO ) + H O
3
3 2
2
Plumbum(II) nitrat PbCO + 2HNO Pb(NO ) + CO + H O
3 3 3 2 2 2
Barium sulphate IS BaCl + H SO BaSO + 2HCl
2
4
4
2
Barium sulfat BaCl + Na SO BaSO + 2NaCl
2 2 4 4
144
Nilam Publication Sdn. Bhd.
07-Chem F4 (3p).indd 144 12/9/2011 5:55:21 PM