Page 4 - COPD - Training Manual - Module 4
P. 4
4.2 Working of Synchrobreathe®
The biasing spring is held under compression until the dust cap has been opened
and as the patient inhales, the resultant flow rate triggers the movement of the
flap. The collapsing of the engine mechanism then causes the biasing spring to
‘force’ the canister downward to fire a dose.
4.3 Synchrobreathe® – simple to use
Synchrobreathe® can be used in three simple steps: shake and open – breathe –
close.
4.4 Lung deposition with Synchrobreathe®
Two lung deposition studies were done with the Synchrobreathe® inhaler – an in
vitro study (a study done in lab to mimic the deposition in the human respiratory
system) and an in-vivo study (a study done in patients).
4.4.1 In vitro lung deposition
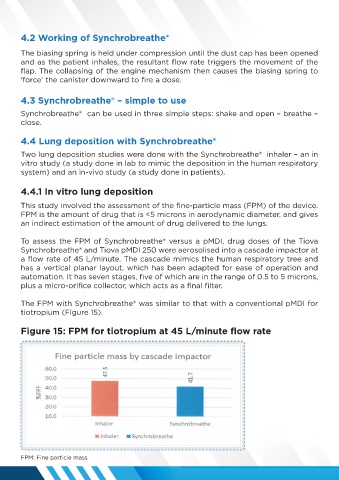
This study involved the assessment of the fine-particle mass (FPM) of the device.
FPM is the amount of drug that is <5 microns in aerodynamic diameter, and gives
an indirect estimation of the amount of drug delivered to the lungs.
To assess the FPM of Synchrobreathe® versus a pMDI, drug doses of the Tiova
Synchrobreathe® and Tiova pMDI 250 were aerosolised into a cascade impactor at
a flow rate of 45 L/minute. The cascade mimics the human respiratory tree and
has a vertical planar layout, which has been adapted for ease of operation and
automation. It has seven stages, five of which are in the range of 0.5 to 5 microns,
plus a micro-orifice collector, which acts as a final filter.
The FPM with Synchrobreathe® was similar to that with a conventional pMDI for
tiotropium (Figure 15).
Figure 15: FPM for tiotropium at 45 L/minute flow rate
FPM: Fine particle mass