Page 5 - COPD - Training Manual - Module 4
P. 5
4.4.2 In vivo study: The gamma study on Synchrobreathe®
Gamma-scintigraphy is a non-invasive method that uses a standardised
radiolabelling technique to assess regional deposition and distribution of
aerosolised drug in the airways.
This prospective, randomised, open-label, cross-over, two-period, single-dose
study aimed to evaluate lung deposition of a radiolabelled active component
when delivered via the Synchrobreathe® inhaler compared with the conventional
pMDI in patients with stable persistent asthma.
Ten stable adult patients with asthma received one pu of radiolabelled (99 mTc)
drug via either Synchrobreathe® or a conventional pMDI (both manufactured by
Cipla Ltd, India) on the first treatment day, and via the alternative device on the
second treatment day.
Subjects of either gender between 18 and 70 years (both inclusive) of age with
physician-diagnosed asthma according to the Global Initiative for Asthma (GINA)
2015 guideline and patients with poor coordination of actuation with inspiration
were included.
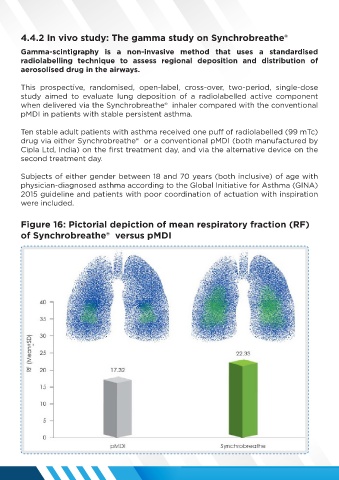
Figure 16: Pictorial depiction of mean respiratory fraction (RF)
of Synchrobreathe® versus pMDI