Page 6 - COPD - Training Manual - Module 4
P. 6
The study results (Figure 16) demonstrated greater lung deposition of inhaled
drug with Synchrobreathe® compared with a pMDI device.
Despite having similar in vitro results when compared with a pMDI, the in vivo
gamma-study shows that in poor coordinators, Synchrobreathe® had greater
drug deposition versus a pMDI.
4.5 Synchrobreathe® – uniform dose delivered with every pu
The Synchrobreathe® inhaler delivers a consistent dose throughout its ‘in-use’ life.
The ‘in-use’ life of Synchrobreathe® is spread over 120 doses. A consistent
delivered dose in the ‘in-use’ life would reflect the reproducibility and reliability of
the device.
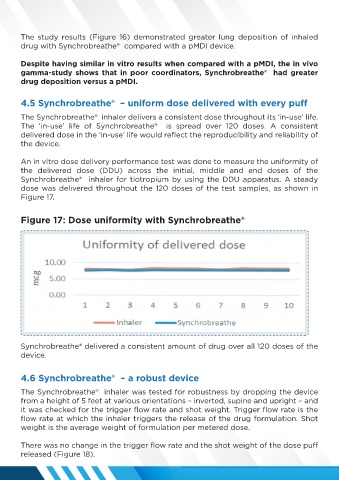
An in vitro dose delivery performance test was done to measure the uniformity of
the delivered dose (DDU) across the initial, middle and end doses of the
Synchrobreathe® inhaler for tiotropium by using the DDU apparatus. A steady
dose was delivered throughout the 120 doses of the test samples, as shown in
Figure 17.
Figure 17: Dose uniformity with Synchrobreathe®
Synchrobreathe® delivered a consistent amount of drug over all 120 doses of the
device.
4.6 Synchrobreathe® – a robust device
The Synchrobreathe® inhaler was tested for robustness by dropping the device
from a height of 5 feet at various orientations – inverted, supine and upright – and
it was checked for the trigger flow rate and shot weight. Trigger flow rate is the
flow rate at which the inhaler triggers the release of the drug formulation. Shot
weight is the average weight of formulation per metered dose.
There was no change in the trigger flow rate and the shot weight of the dose pu
released (Figure 18).