Page 256 - APPLIED PROCESS DESIGN FOR CHEMICAL AND PETROCHEMICAL PLANTS, Volume 1, 3rd Edition
P. 256
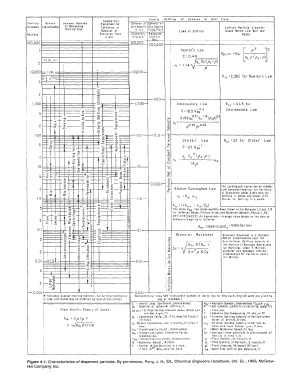
Grovi ty Sett Ii ng Of Spheres in Slill Fluid
Cc mme rcrc l
Port icle General Common Mel hods Equipment for Spheres of Spheres of
Diameter Classification of Mea,uring Collection or Unit Densify Any Densily Crilical Par ti cit Diameter
Port icol Si,e Removal ot i n Air in An1 Fluid Lows of Sel lling Above Which Law Will Not
Microns Particles from Diomet er, Reynolds Apply
o Gos Microns Number
URe
100,000 ; 100,000 - 200,000 �, r
5 New Ion's Law t
glp(ps-pl,
Op,crit = Ker
C=0.44
-lin.- )gLOp{p,-p)
2
; u t : 1.74
: f
10,000 =I cm. . I -10,000-
-I Ker =2,360 for Newton's Law
= in.
5 4 I
I
"' �
.,
2 ..CJ
' . . : E ' I 500-
. .
0
u
1,000 .<= I 1,000- Intermediate Law Kc, : 43.5 far
"'
.,- "' -.,,- C = 18.5 Nri.°"6 Intermediate Law
-�-
,- ·-
,-
>-- :;::
5 ____ w_
- ., -2-
- "'- C> 0 - en -�- ·- 0.153gt71 Op l.14(fsf')0.7.
>-
-- - "' ---- - -
..
>-
u u, =
0
a.,
·;:.
�
0.
0
29
2 -'- o::- -.�----:.;- -- � p° f' 0.43
a.,-
f--->
(J) ·en e � (J)
·- <!) 2 >
> -
100 ' �-�:- 100-
-2.0- 0
I - ' � ���� "' Stokes ' Law Ker : 33 for Stokes ' Law
E
- >-
.... �
....
- 0 ' en � - �� �
5 - 0. ' I- 1
- '"'en "' I - ,_- 0. C = 2 4 N R;
0
0
E
C>
- "' .s I - � " rr r � 2
·- �
0
·-
0
- '
2 -;; 2 ' - �r "' gl Dp (fs-f)
"' .,
"' ut =
- "' '
0
}. w '-' I !: ' u »» = u,s
�
10 I I ' LL I 10- -
.,
§ '
"'O 0
-
,-- (/} "'c,: -
-
- ... ..... c,,
5-6 .... if ., :*� I �- 1-.L �:t: �
...
·-
I
CO I
-
I ·-
"'
0. ' ; .. I ., - T -
..c
_-o
.0
0 0 �� � �i 0.0001- u
...
u
2 >-- --- - -� �r - U o O c.. Stokes-Cunningham Law .The Cunningham Correction on Stokes•
Low Becomes lrnper tcnt for Particles
--U
0
u
"'
I�
:::;; ·- C.. I cn o> c.. = Km u ts of Diameters Under 3 Microns for
:::;;
Settling in Gases ond Under 0.01
I
1.0 I I .,;: . 1.0- ut Micron for Sellling in Liquids.
u
•
-
I
(I)
5 a., )( t� �,,_--.o - Km = I +Km, ( >. m I Op)
The Value Km, hos Experimentally been Shown to lie Between 1.3 and 2.3
Cl.,--,-�
r-"
I
� g- �1- :,=ff=:. for Different Goses I Particle Sizes I and Materials {Wasser I Physik.Z. 34 1
1
�
I
-
-Q.J-- Q)- r---1- o "= -r- E 11- I-· �
2 -=, �e- �I- � � -1- ] .i. ..... ., i � 257- 278 [1933 )). An Approximate Average Value Booed on lhe Doto of
E
""'
Millikan is Empiricolly Given by
0
�
u, en � Q) ...... '* a, I �
(..) ;:) I C: - I � Km, =1.644 +0.552e -(0.656 Op/>.m)
::::,
0.1 � I 7 t ·� ..... 0 0.1
u .
* .!:: a. - <,.) ·-
.....
�� j:Q) � g ... !: : Brownian Movement Brownian Movement is o Random
5 OW 01 <I - ., "' Motion Superimposed upon 1ne
"'O
w
=>
<,.)
� I-�-*·- -w " Grovitotionol Settling Velocity of
�
�
"'
u
the Particle it Becomes Appreciobe
.� ��-.2 "' t:i.x = 4gc RT Km t for Particles under 3 Microns
0
E Q) "u :;; Diameter end Becomes Entirely
2 � 1---U-c, I "' 3 .r 2 f N Op Predominant for Particles Under
::::!
- 0 ::::, = zs t I <!) 0 0.1 Micron.
::::,
0.01 IOOA >, 0.01- �
0
� 0:: I . ....!
• ,.,, I t . I I :::;;
>
5 ' x o.>
0
"'
o.>
-- Q) ' t -
�:5
2�,,_o t I ·-
u
o�
....! 0 ' I ' I
0
,:::1: Q_
t-- 0.001 ' 0.001
+ Furnishes Average particle Diameter, but no Size 01sfr1bufion Nomencloture: I Any Self-Consiste11t System of Units moy be Employedj English units are given by
x Size DistrJbut1on may be Obtained by Special Calibration way of E,ampte.)
c : Overall Oroq Coefficient, Dlmensionless NRc = Reynolrls Number ,Dimensionless =Dppl" E/P,
Op :: D:Ometer of Spherical Particle, ft. R = Gos Constant, 1,546 (ff.-lb. Force) lib. mole)( °F)
From Kinetic Theory of Gases: Dp crit =Critical Particle Diameter Abo¥e Which LOW I =Time, sec.
will Not Apply, ft. T = Absolute Gos Temperature °F abs.,or 0 R
1
)..,. = 3 fl p v gc = Conversion Foe tor 1 32.17 (lb.moss /lb. Forcei I •1 = Terminal Settling Velocity of Particle Under
It 1./sec.)
Action of Gravity, ft./sec.
gc DLocol Acceleration due to Gravity ,(ft.)/lsec.) 'ts = Terminal Settling verocity of Particle as
v =JBg, RT /irM ls"·.l Colculated from Stokes' Law I ft./uc.
Ker = Proportional i ly Factor I Dimensionless v = Mean Moleculor Speed, fl./sec.
Km = Stokes-Cunningham Correction Factor, Ax = Average Linear Amplitude or Oisplocement of
Dimensionless Particle in Time t I ff.
Kme = Proportionolify Factor, Dimensionless p = Fluid Density, lb. moss/cu. fr.
L N = Number ot Gos Molecules in o mole I •m = Mean Free Poth of Gos Molecules ,ft.
M = Molecular Weight, lb./mole
P, =True Density of Porticle,lb.mass/cu.ft.
µ = Fluid Viscosity, (lb. mos,)/ (ft.Ilse,)
2.76 x 102t' f'olecule; /lb. mole
Figure 4-1. Characteristics of dispersed particles. By permission, Perry, J. H., Ed., Chemical Engineers Handbook, 3rd. Ed., 1950, McGraw-
Hill Company, Inc.