Page 7 - Modul A+ Kimia Tg 4_Pan Asia Publications
P. 7
Uji Kendiri 3.1
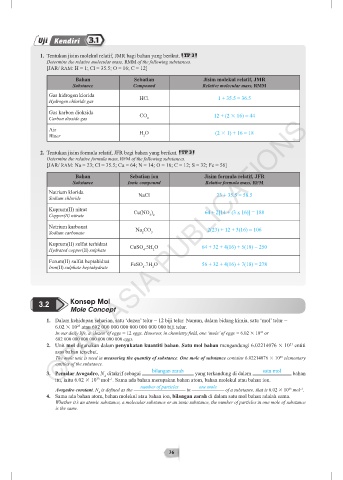
1. Tentukan jisim molekul relatif, JMR bagi bahan yang berikut. TP 3
Determine the relative molecular mass, RMM of the following substances.
[JAR/ RAM: H = 1; Cl = 35.5; O = 16; C = 12]
Bahan Sebatian Jisim molekul relatif, JMR
Substance Compound Relative molecular mass, RMM
Gas hidrogen klorida
Hydrogen chloride gas HCl 1 + 35.5 = 36.5
Gas karbon dioksida
Carbon dioxide gas CO 2 12 + (2 16) = 44
©PAN ASIA PUBLICATIONS
Air
Water H O (2 1) + 16 = 18
2
2. Tentukan jisim formula relatif, JFR bagi bahan yang berikut. TP 3
Determine the relative formula mass, RFM of the following substances.
[JAR/ RAM: Na = 23; Cl = 35.5; Cu = 64; N = 14; O = 16; C = 12; S = 32; Fe = 56]
Bahan Sebatian ion Jisim formula relatif, JFR
Substance Ionic compound Relative formula mass, RFM
Natrium klorida NaCl 23 + 35.5 = 58.5
Sodium chloride
Kuprum(II) nitrat Cu(NO ) 64 + 2[14 + (3 x 16)] = 188
Copper(II) nitrate 3 2
Natrium karbonat Na CO 2(23) + 12 + 3(16) = 106
Sodium carbonate 2 3
Kuprum(II) sulfat terhidrat CuSO .5H O 64 + 32 + 4(16) + 5(18) = 250
Hydrated copper(II) sulphate 4 2
Ferum(II) sulfat heptahidrat FeSO .7H O 56 + 32 + 4(16) + 7(18) = 278
Iron(II) sulphate heptahydrate 4 2
3.2 Konsep Mol
Mole Concept
1. Dalam kehidupan seharian, satu ‘dozen’ telur = 12 biji telur. Namun, dalam bidang kimia, satu ‘mol’ telur =
6.02 10 atau 602 000 000 000 000 000 000 000 biji telur.
23
In our daily life, a ‘dozen’ of eggs = 12 eggs. However, in chemistry field, one ‘mole’ of eggs = 6.02 10 or
23
602 000 000 000 000 000 000 000 eggs.
2. Unit mol digunakan dalam penyukatan kuantiti bahan. Satu mol bahan mengandungi 6.02214076 10 entiti
23
asas bahan tersebut.
The mole unit is used in measuring the quantity of substance. One mole of substance contains 6.02214076 10 elementary
23
entities of the substance.
3. Pemalar Avogadro, N ditakrif sebagai bilangan zarah yang terkandung di dalam satu mol bahan
A
itu, iaitu 6.02 10 mol . Sama ada bahan merupakan bahan atom, bahan molekul atau bahan ion.
–1
23
number of particles one mole
Avogadro constant, N is defined as the in of a substance, that is 6.02 10 mol .
23
–1
A
4. Sama ada bahan atom, bahan molekul atau bahan ion, bilangan zarah di dalam satu mol bahan adalah sama.
Whether it’s an atomic substance, a molecular substance or an ionic substance, the number of particles in one mole of substance
is the same.
36