Page 119 - Basic Principles of Textile Coloration
P. 119
108 PROTEIN FIBRES
the requirement for enantiomerism and optical activity. Hydrolysis, or enzyme
digestion, of a protein breaks all the amide groups in the polymer and produces the
constituent amino acids, which can be isolated and identified.
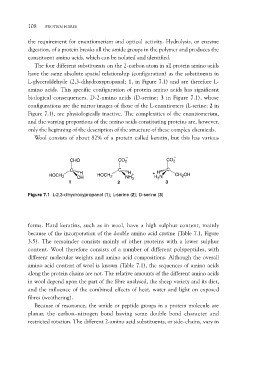
The four different substituents on the 2-carbon atom in all protein amino acids
have the same absolute spatial relationship (configuration) as the substituents in
L-glyceraldehyde (2,3-dihydroxypropanal; 1, in Figure 7.1) and are therefore L-
amino acids. This specific configuration of protein amino acids has significant
biological consequences. D-2-amino acids (D-serine; 3 in Figure 7.1), whose
configurations are the mirror images of those of the L-enantiomers (L-serine; 2 in
Figure 7.1), are physiologically inactive. The complexities of the enantiomerism,
and the varying proportions of the amino acids constituting proteins are, however,
only the beginning of the description of the structure of these complex chemicals.
Wool consists of about 82% of a protein called keratin, but this has various
CHO CO2 CO2
HOCH2 CH HOCH2 CH HC CH2OH
OH NH3 H3N
1 2 3
Figure 7.1 L-2,3-dihydroxypropanal (1); L-serine (2); D-serine (3)
forms. Hard keratins, such as in wool, have a high sulphur content, mainly
because of the incorporation of the double amino acid cystine (Table 7.1, Figure
3.5). The remainder consists mainly of other proteins with a lower sulphur
content. Wool therefore consists of a number of different polypeptides, with
different molecular weights and amino acid compositions. Although the overall
amino acid content of wool is known (Table 7.1), the sequences of amino acids
along the protein chains are not. The relative amounts of the different amino acids
in wool depend upon the part of the fibre analysed, the sheep variety and its diet,
and the influence of the combined effects of heat, water and light on exposed
fibres (weathering).
Because of resonance, the amide or peptide groups in a protein molecule are
planar, the carbon–nitrogen bond having some double bond character and
restricted rotation. The different 2-amino acid substituents, or side-chains, vary in