Page 260 - Basic Principles of Textile Coloration
P. 260
MECHANISM OF WOOL DYEING 249
some of these will accept protons to form ammonium ions. These cationic groups
are capable of binding dye anions by ionic attraction. Practical dyeing with acid
dyes, even in the presence of sulphuric acid, only involves a very small fraction of
the available cationic sites. Saturation of all such potential sites in acidic wool
with a simple acid dye such as Orange II (Figure 1.1, molecular weight
350 g mol–1) corresponds to almost a 30% owf dyeing.
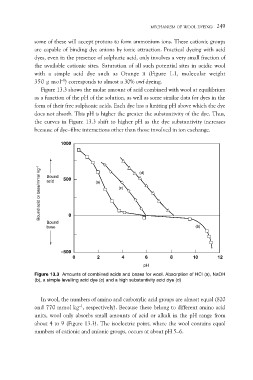
Figure 13.3 shows the molar amount of acid combined with wool at equilibrium
as a function of the pH of the solution, as well as some similar data for dyes in the
form of their free sulphonic acids. Each dye has a limiting pH above which the dye
does not absorb. This pH is higher the greater the substantivity of the dye. Thus,
the curves in Figure 13.3 shift to higher pH as the dye substantivity increases
because of dye–fibre interactions other than those involved in ion exchange.
1000
Bound acid or base/mmol kg–1 Bound 500 (d)
acid
(a)
(c)
0
Bound (b)
base
–500
0 2 4 6 8 10 12
pH
Figure 13.3 Amounts of combined acids and bases for wool. Absorption of HCl (a), NaOH
(b), a simple levelling acid dye (c) and a high substantivity acid dye (d)
In wool, the numbers of amino and carboxylic acid groups are almost equal (820
and 770 mmol kg–1, respectively). Because these belong to different amino acid
units, wool only absorbs small amounts of acid or alkali in the pH range from
about 4 to 9 (Figure 13.3). The isoelectric point, where the wool contains equal
numbers of cationic and anionic groups, occurs at about pH 5–6.