Page 391 - Basic Principles of Textile Coloration
P. 391
380 VAT DYES N
C NH2
N
S
S
NS N
S OO S N
SS
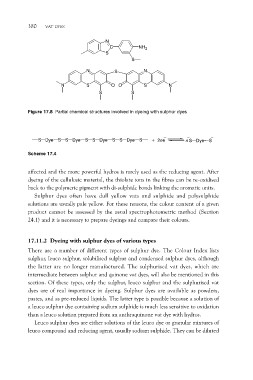
Figure 17.8 Partial chemical structures involved in dyeing with sulphur dyes
S Dye S S Dye S S Dye S S Dye S + 2ne n S Dye S
Scheme 17.4
affected and the more powerful hydros is rarely used as the reducing agent. After
dyeing of the cellulosic material, the thiolate ions in the fibres can be re-oxidised
back to the polymeric pigment with di-sulphide bonds linking the aromatic units.
Sulphur dyes often have dull yellow vats and sulphide and polysulphide
solutions are usually pale yellow. For these reasons, the colour content of a given
product cannot be assessed by the usual spectrophotometric method (Section
24.1) and it is necessary to prepare dyeings and compare their colours.
17.11.2 Dyeing with sulphur dyes of various types
There are a number of different types of sulphur dye. The Colour Index lists
sulphur, leuco sulphur, solubilised sulphur and condensed sulphur dyes, although
the latter are no longer manufactured. The sulphurised vat dyes, which are
intermediate between sulphur and quinone vat dyes, will also be mentioned in this
section. Of these types, only the sulphur, leuco sulphur and the sulphurised vat
dyes are of real importance in dyeing. Sulphur dyes are available as powders,
pastes, and as pre-reduced liquids. The latter type is possible because a solution of
a leuco sulphur dye containing sodium sulphide is much less sensitive to oxidation
than a leuco solution prepared from an anthraquinone vat dye with hydros.
Leuco sulphur dyes are either solutions of the leuco dye or granular mixtures of
leuco compound and reducing agent, usually sodium sulphide. They can be diluted