Page 419 - Basic Principles of Textile Coloration
P. 419
408 DYES SYNTHESISED IN THE FIBRE
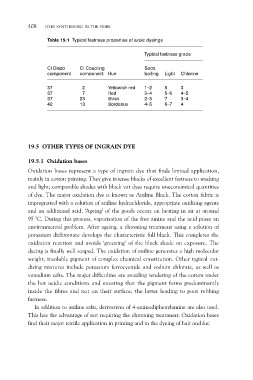
Table 19.1 Typical fastness properties of azoic dyeings
Typical fastness grade
CI Diazo CI Coupling Soda
boiling Light Chlorine
component component Hue
37 2 Yellowish red 1–2 5 3
37 7 Red 3–4 5–6 4–5
37 23 Black 2–3 7 3–4
42 13 Bordeaux 4–5 6–7 4
19.5 OTHER TYPES OF INGRAIN DYE
19.5.1 Oxidation bases
Oxidation bases represent a type of ingrain dye that finds limited application,
mainly in cotton printing. They give intense blacks of excellent fastness to washing
and light; comparable shades with black vat dyes require uneconomical quantities
of dye. The major oxidation dye is known as Aniline Black. The cotton fabric is
impregnated with a solution of aniline hydrochloride, appropriate oxidising agents
and an additional acid. ‘Ageing’ of the goods occurs on heating in air at around
95 °C. During this process, vaporisation of the free amine and the acid poses an
environmental problem. After ageing, a chroming treatment using a solution of
potassium dichromate develops the characteristic full black. This completes the
oxidation reaction and avoids ‘greening’ of the black shade on exposure. The
dyeing is finally well soaped. The oxidation of aniline generates a high molecular
weight, insoluble pigment of complex chemical constitution. Other typical oxi-
dising mixtures include potassium ferrocyanide and sodium chlorate, as well as
vanadium salts. The major difficulties are avoiding tendering of the cotton under
the hot acidic conditions and ensuring that the pigment forms predominantly
inside the fibres and not on their surface, the latter leading to poor rubbing
fastness.
In addition to aniline salts, derivatives of 4-aminodiphenylamine are also used.
This has the advantage of not requiring the chroming treatment. Oxidation bases
find their major textile application in printing and in the dyeing of hair and fur.