Page 414 - Basic Principles of Textile Coloration
P. 414
AZOIC DYES 403
amines used are simple substituted aniline derivatives with no ionic substituents.
More complex amines give violet and blue shades.
The so-called Fast Colour Bases require diazotisation. This usually involves
reaction of the primary aromatic amine in acidic solution or dispersion with
sodium nitrite, at or below room temperature. Successful diazotisation requires
careful weighing of all the chemicals and regard for the supplier’s
recommendations. Diazotisation of a primary aromatic amine is often difficult and
solutions of diazonium ions are inherently unstable. They undergo decomposition
even at low temperature and particularly on exposure to light. Storing prepared
diazonium ion solutions is not usually possible.
Because of the many difficulties that arise in the diazotisation reaction, dye
manufacturers market a variety of stabilised diazonium components. These
diazonium salts are soluble in water and are then immediately ready for the
coupling reaction. Some of these products are stable diazonium ion salts such as
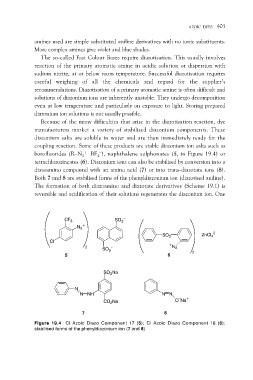
borofluorides (R–N2+ BF4–), naphthalene sulphonates (5, in Figure 19.4) or
tetrachlorozincates (6). Diazonium ions can also be stabilised by conversion into a
diazoamino compound with an amino acid (7) or into trans-diazotate ions (8).
Both 7 and 8 are stabilised forms of the phenyldiazonium ion (diazotised aniline).
The formation of both diazoamino and diazotate derivatives (Scheme 19.1) is
reversible and acidification of their solutions regenerates the diazonium ion. One
CF3 SO3–
N2+ SO3–
Cl 2 SO2 ZnCl42–
5 +N2
6 2
SO3Na
N NN
N NH O–Na+
CO2Na
78
Figure 19.4 CI Azoic Diazo Component 17 (5); CI Azoic Diazo Component 18 (6);
stabilised forms of the phenyldiazonium ion (7 and 8)