Page 89 - Basic Principles of Textile Coloration
P. 89
78 NATURAL CELLULOSIC FIBRES
prevents crystallinity, is much more accessible to water and the unbranched
amylose is soluble.
Cellulose is susceptible to hydrolysis by hot dilute solutions of mineral acids.
Initially, this breaks the polymer chain at the oxygen atoms between C-1 and C-4
of adjacent glucose units, causing a dramatic drop in DP, and a loss of fibre
strength called tendering. The lower molecular weight insoluble celluloses
obtained from such partial hydrolysis are called hydrocelluloses. If hydrolysis
continues, the eventual product is the soluble monomer glucose. Because of this
sensitivity towards acids, cellulose fibres are never dyed at a pH below 3–4. For the
same reason, wet cellulose must never be heated, dried or stored when it contains
residues of mineral acids. After treatment of a cellulosic material with an alkaline
solution, acetic acid is the preferred acid for neutralisation. This is a weak acid
that will vaporise during drying before it concentrates to the point at which the
pH becomes low enough to result in hydrocellulose formation.
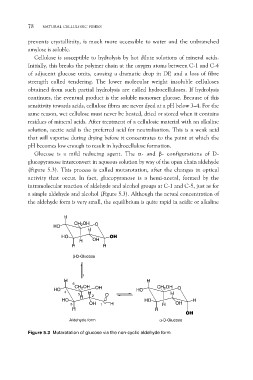
Glucose is a mild reducing agent. The a- and b- configurations of D-
glucopyranose interconvert in aqueous solution by way of the open chain aldehyde
(Figure 5.3). This process is called mutarotation, after the changes in optical
activity that occur. In fact, glucopyranose is a hemi-acetal, formed by the
intramolecular reaction of aldehyde and alcohol groups at C-1 and C-5, just as for
a simple aldehyde and alcohol (Figure 5.3). Although the actual concentration of
the aldehyde form is very small, the equilibrium is quite rapid in acidic or alkaline
H
HO CH2OH O
H
HO OH OH
H H
H
β-D-Glucose
H 6 H
HO 4 CH2OH OH HO CH2OH O
HO H
5 H O
H 2
3 OH C H HO OH H
H OH
1
H
H
Aldehyde form α-D-Glucose
Figure 5.3 Mutarotation of glucose via the non-cyclic aldehyde form