Page 91 - Basic Principles of Textile Coloration
P. 91
80 NATURAL CELLULOSIC FIBRES
present, however, the cellulose will contain negatively charged carboxylate groups
at pH values above 4–5 and these can bind cationic dye molecules. Staining of
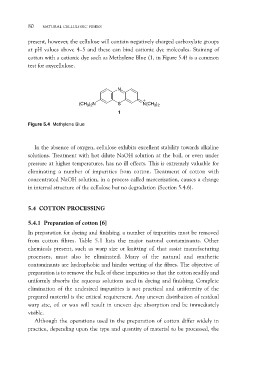
cotton with a cationic dye such as Methylene Blue (1, in Figure 5.4) is a common
test for oxycellulose.
N
(CH3)2N S N(CH3)2
1
Figure 5.4 Methylene Blue
In the absence of oxygen, cellulose exhibits excellent stability towards alkaline
solutions. Treatment with hot dilute NaOH solution at the boil, or even under
pressure at higher temperatures, has no ill effects. This is extremely valuable for
eliminating a number of impurities from cotton. Treatment of cotton with
concentrated NaOH solution, in a process called mercerisation, causes a change
in internal structure of the cellulose but no degradation (Section 5.4.6).
5.4 COTTON PROCESSING
5.4.1 Preparation of cotton [6]
In preparation for dyeing and finishing, a number of impurities must be removed
from cotton fibres. Table 5.1 lists the major natural contaminants. Other
chemicals present, such as warp size or knitting oil that assist manufacturing
processes, must also be eliminated. Many of the natural and synthetic
contaminants are hydrophobic and hinder wetting of the fibres. The objective of
preparation is to remove the bulk of these impurities so that the cotton readily and
uniformly absorbs the aqueous solutions used in dyeing and finishing. Complete
elimination of the undesired impurities is not practical and uniformity of the
prepared material is the critical requirement. Any uneven distribution of residual
warp size, oil or wax will result in uneven dye absorption and be immediately
visible.
Although the operations used in the preparation of cotton differ widely in
practice, depending upon the type and quantity of material to be processed, the