Page 58 - fbkCardioDiabetes_2017
P. 58
34 Cardio Diabetes Medicine 2017
nary revascularization by 22 %,P=<0.001. There was
no significant decrease in all cause or CV mortality.
Alirocumab
This is approved for clinical use in USA and is com-
mercially available as 1 ml pen containing 75 or 150
mg. It is given in doses in 150 mg. biweekly sc. It was
evaluated in ODYSSEY Global Programme which in-
cluded 11 trials and roughly has 22,000 patients
Bococizumab
The SPIRE 1 and 2 with Bococizumab was premature-
ly terminated because of presence of neutralizing
antibody in 29% and antidrug antibodies in 48% of
patients taking the drug. This is perhaps because Bo-
cocizumab is partially murine monoclonal antibody
unlike Evolocumab and Alirocumab which are fully
humanized monoclonal antibodies. The incidence of
antidrug antibody and neutralizing antibody to vari-
ous PCSK9 inhibitors are mentioned in Table 5.
1 Failure to achieve LDL-C goals despite optimal
doses of statins in patients with ASCVD
2 Statin intolerance
3 Familial hypercholesterolemia
Table 5: Indications of PCSK9
Given the lack of long-term safety and efficacy data
on these agents, they are not recommended for use
for primary prevention except in patients with familial
hypercholesterolemia. The data of PCSK9 for primary
prevention like high risk diabetics is yet to evolve out
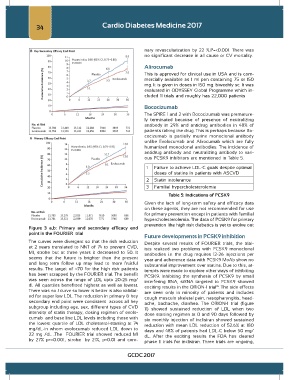
Figure 3 a,b: Primary and secondary efficacy end
point in the FOURIER trial
Future developments in PCSK9 inhibition
The curves were divergent so that the risk reduction Despite several results of FOURIER trials, the trial-
at 2 years translated to NNT of 74 to prevent CVD, ists realized two problems with PCSK9 monoclonal
MI, stroke but at three years it decreased to 50. It antibodies i.e. the drug requires 12-26 injections per
seems that the future is brighter than the present year and adherence data with PCSK9 MoAb show no
and long term follow up may lead to more fruitful substantial improvement over statins. Due to this, at-
results. The target of <70 for the high risk patients tempts were made to explore other ways of inhibiting
has been scrapped by the FOURIER trial. The benefit PCSK9. Inhibiting the synthesis of PCSK9 by small
was seen across the range of LDL upto 20-25 mg/ interfering RNA, siRNA targeted to PCSK9 showed
dl. All quartiles benefitted highest as well as lowest. exciting results in the ORION-1 trial . The side effects
15
There was no J curve so lower is better is also validat- are seen only in minority of patients and includes
ed for super low LDL. The reduction in primary & key cough musculo skeletal pain, nasopharyngitis, head-
secondary end point were consistent across all key ache, backache, diarrhea. The ORION-1 trial (figure
subgroup including age, sex, different types of CVD 11) showed sustained reduction of LDL when two
intensity of statin therapy, dosing regimen of evolo- dose starting regimen at 0 and 90 days followed by
cumab and base line LDL levels including those with six monthly injection of inclisiran showed sustained
the lowest quartile of LDL cholesterol-starting at 74 reduction with mean LDL reduction of 52.6% at 180
mg/dL-in whom evolocumab reduced LDL down to days and 48% of patients had LDL-C below 50 mg/
22 mg /dL .The FOURIER trial showed reduced MI dL. After the exciting results the FDA has cleared
by 27% p=<0.001, stroke by 21%, p=0.01 and coro- phase II trials for inclisiran. Three trials are ongoing,
GCDC 2017