Page 126 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 126
92 PART 1: An Overview of the Approach to and Organization of Critical Care
admission. The TS and the RTS are derived from the Triage Index. low rates of serious bleeding (1.2% vs 1%). On October 25, 2011, Eli
42
43
In the part of the RTS used for triage, the T-RTS, specific decision rules Lilly and Company withdrew rhAPC from the market worldwide. In
are proposed to indicate appropriate transfer to a trauma center. These PROWESS SHOCK, the observed pooled mortality was much lower
43
rules are based on the score and the GCS score. There are at least two than expected, lower than in PROWESS, which enrolled a broader
caveats regarding use of scoring systems to guide ICU triage decisions. population of severe sepsis. The low mortality rates observed in
First, a patient who could be admitted to the ICU who has a very low PROWESS SHOCK may be explained in part by recent advances
probability of mortality estimated by the MPM , might in fact have a in the management of septic shock and in part by the selection of lower
0
higher actual probability of mortality if ICU admission were denied, risk patients.
20
because outcome could be adversely affected by ward admission and the In support of this approach (ie, use of APACHE II to identify high-
associated lower intensity of monitoring and treatment. Second, physi- risk patients), a cost-effectiveness analysis by Manns and coworkers
68
cians tend to underestimate mortality in low-risk patients. Thus scoring found that rhAPC is relatively cost-effective when targeted to patients
systems can be more accurate than clinician judgment for risk estimate with severe sepsis, greater severity of illness (an APACHE II score of 25
of low-risk patients. 31 or more), and a reasonable life expectancy if they survive the episode
A novel use of severity of illness scoring systems is in patient selection of sepsis. In a second cost-effectiveness analysis by Angus and cowork-
for specific therapies. The initial approval of a now withdrawn therapy for ers, rhAPC cost $27,400 per quality-adjusted life-year when limited to
severe sepsis, recombinant human activated protein C (rhAPC; drotre- patients with an APACHE II score ≥25, and was cost-ineffective when
cogin alfa), was an example. In the original pivotal multicenter RCT limited to patients with a score <25. Manns and coworkers concluded,
69
(PROWESS trial), rhAPC significantly decreased mortality compared to “given the discrepancy between the published study results and their
placebo in treatment of severe sepsis. Treatment with rhAPC activated analysis, it would be reasonable to restrict the use of activated protein C
40
was associated with a reduction in the relative risk of death of 19.4% (95% to patients with an APACHE II score of 25 or more until convincing evi-
confidence interval [CI], 6.6 to 30.5) and an absolute reduction in the risk dence of effectiveness and cost-effectiveness in patients with less severe
of death of 6.1% (31% vs 25%, p = 0.005). The number needed to treat illness becomes available.” 68
(NNT) with rhAPC was 16 to save one life. A post hoc analysis of the There are several criticisms of using baseline APACHE II scores in
study data performed by the FDA reported a differential benefit accord- individual patients to guide therapy. First, the PROWESS trial was
70
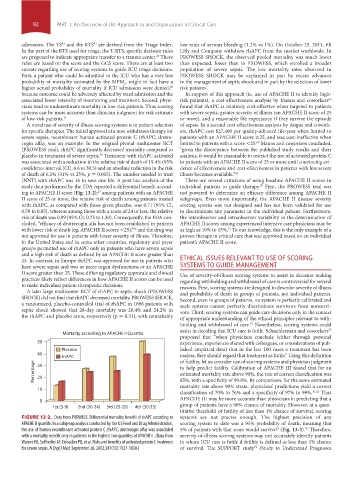
ing to APACHE II score (Fig. 13-2); among patients with an APACHE not powered to determine an efficacy difference among APACHE II
67
II score of 25 or more, the relative risk of death among patients treated subgroups. Even more importantly, the APACHE II disease severity
with rhAPC, as compared with those given placebo, was 0.71 (95% CI, scoring system was not designed and has not been validated for use
0.59 to 0.85), whereas among those with a score of 24 or less, the relative to discriminate any parameter in the individual patient. Furthermore,
risk of death was 0.99 (95% CI, 0.75 to 1.30). Consequently, the FDA con- the interobserver and intraobserver variability in the determination of
cluded, “efficacy of drotrecogin alfa has not been established in patients APACHE II scores among experienced intensive care physicians may be
with lower risk of death (eg, APACHE II scores <25),” and the drug was as high as 10% to 15%. To our knowledge, this is the only example of a
71
66
not approved for use in patients with lower severity of illness. Therefore, proven therapy in critical care that was approved based on an individual
in the United States and in some other countries, regulatory and payer patient’s APACHE II score.
groups permitted use of rhAPC only in patients who have severe sepsis
and a high risk of death as defined by an APACHE II score greater than
25. In contrast, in Europe rhAPC was approved for use in patients who ETHICAL ISSUES RELEVANT TO USE OF SCORING
have severe sepsis and two or more organ dysfunctions or an APACHE SYSTEMS TO GUIDE MANAGEMENT
II score greater than 25. These differing regulatory approvals and clinical Use of severity-of-illness scoring systems to assist in decision making
practices likely reflect differences in how APACHE II scores can be used regarding withholding and withdrawal of care is controversial for several
to make individual patient therapeutic decisions. reasons. First, scoring systems are designed to describe severity of illness
A later large multicenter RCT of rhAPC in septic shock (PROWESS and probability of death in groups of patients, not individual patients.
SHOCK) did not find that rhAPC decreased mortality. PROWESS SHOCK, Second, even in groups of patients, no system is perfectly calibrated and
a randomized, placebo-controlled trial of rhAPC in 1696 patients with such systems cannot perfectly discriminate survivors from nonsurvi-
septic shock showed that 28-day mortality was 26.4% and 24.2% in vors. Third, scoring systems can guide care decisions only in the context
the rhAPC and placebo arms, respectively (p = 0.31), with remarkably of appropriate understanding of the ethical principles relevant to with-
holding and withdrawal of care. Nonetheless, scoring systems could
72
assist in deciding that ICU care is futile. Schneiderman and coworkers
73
Mortality according to APACHE II Quartile
proposed that “when physicians conclude (either through personal
50 experience, experiences shared with colleagues, or considerations of pub-
Placebo lished empirical data) that in the last 100 cases a treatment has been
40 useless, they should regard that treatment as futile.” Using this definition
rhAPC of futility, let us consider use of scoring systems and physician judgment
Percentage 30 to help predict futility. Calibration of APACHE III found that for an
estimated mortality rate above 90%, the rate of correct classification was
20
85%, with a specificity of 99.8%. By comparison, for the same estimated
mortality rate above 90% strata, physicians’ predictions yield a correct
10
classification of 70% to 76% and a specificity of 97% to 99%. 31,32 Thus
APACHE III may be more accurate than physicians in predicting that a
0 group of patients have a 90% chance of mortality. However, at a quan-
1st (3-9) 2nd (20-24) 3rd (25-29) 4th (30-53)
titative threshold of futility of less than 1% chance of survival, scoring
FIGURE 13-2. Data from PROWESS. Differential mortality benefit of rhAPC according to systems are not precise enough. The highest precision of any
APACHE II quartile. In a subgroup analysis conducted by the US Food and Drug Administration, scoring system to date was a 95% probability of death, meaning that
28
76
the use of human recombinant activated protein C (rhAPC; drotrecogin alfa) was associated 5% of patients with that score would survive (Fig. 13-3). Therefore,
with a mortality benefit only in patients in the highest two quartiles of APACHE II. (Data from severity-of-illness scoring systems may not accurately identify patients
Warren HS, Suffredini AF, Eichacker PQ, et al. Risks and benefits of activated protein C treatment in whom ICU care is futile if futility is defined as less than 1% chance
for severe sepsis. N Engl J Med. September 26, 2002;347(13):1027-1030.) of survival. The SUPPORT study (Study to Understand Prognoses
74
Section01.indd 92 1/22/2015 9:37:27 AM