Page 629 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 629
448 PART 4: Pulmonary Disorders
frequencies (∼3-15 Hz), so as to avoid tidal overstretch and recruitment/ ECLS. In this trial, 180 patients were randomized to receive venous-
137
derecruitment. Preliminary studies in children and adults appeared venous ECMO or conventional ventilation. Patients randomized to ECLS
131
130
to support these theoretical advantages and suggested that HFO was at were transferred to and treated in a single reference center. Of the patients
least as safe as conventional ventilator strategies and that it was effective transferred, 25% did not receive ECMO (improved or died shortly after
in improving oxygenation. However, these studies were hampered by transfer). The group that were transferred to the center of excellence had
their small size and the fact that the control groups likely did not repre- better 6 months survival compared to control patients who were treated in
sent the current standard of care, namely V <6 mL/kg PBW. In the face regional hospitals where the “best practice” for mechanical ventilation was
t
of persuasive biological rationale and promising preliminary trials, the left to the discretion of the treating physicians. The trial has been criticized
132
results of the OSCILLATE and OSCAR trials were surprising and disap- because all of the ECMO patients went to a specialized center, whereas the
pointing. 133,134 Both trials compared HFO to a lung-protective strategy control group were treated in multiple nonspecialized hospitals. As the side
that employed low tidal volume and higher PEEP levels to fully recruit effects of various types of ECMO are decreasing, this mode of gas exchange
the lung. In the UK study, 398 patients were randomized to HFO and may prove to be very useful.
397 patients to a conventional lung-protective strategy. There was no dif-
ference in mortality between the two groups. Death at 30 days occurred SUMMARY
in 42% in the HFO group compared to 41% in the conventional ventila-
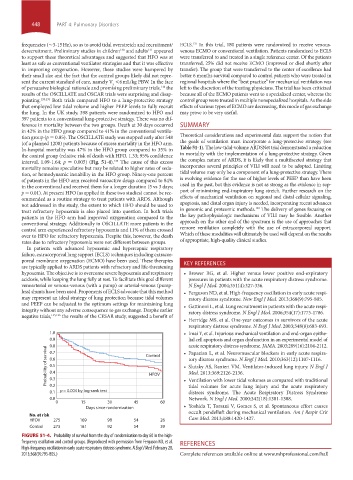
tion group (p = 0.85). The OSCILLATE study was stopped early after 548 Theoretical considerations and experimental data support the notion that
(of a planned 1200) patients because of excess mortality in the HFO arm. the goals of ventilation must incorporate a lung-protective strategy (see
In-hospital mortality was 47% in the HFO group compared to 35% in Table 51-1). The low-tidal-volume ARDSNet trial demonstrated a reduction
the control group (relative risk of death with HFO, 1.33; 95% confidence in mortality with the implementation of a lung-protective strategy. Given
interval, 1.09-1.64; p = 0.005) (Fig. 51-4). The cause of this excess the complex nature of ARDS, it is likely that a multifaceted strategy that
133
mortality remains speculative but may be related to higher rates of seda- incorporates several principles of VILI will need to be adopted. Limiting
tion, or hemodynamic instability in the HFO group. Ninety-one percent tidal volume may only be a component of a lung-protective strategy. There
of patients in the HFO arm received vasoactive drugs compared to 84% is evolving evidence for the use of higher levels of PEEP than have been
in the conventional and received them for a longer duration (5 vs 3 days; used in the past, but this evidence is not as strong as the evidence in sup-
p = 0.01). At present HFO (as applied in these two studies) cannot be rec- port of minimizing end-inspiratory lung stretch. Further research on the
ommended as a routine strategy to treat patients with ARDS. Although effects of mechanical ventilation on regional and distal cellular signaling,
not addressed in the study, the extent to which HFO should be used to apoptosis, and distal organ injury is needed, incorporating recent advances
138
treat refractory hypoxemia is also placed into question. In both trials in genomic and proteomic methods. The delivery of genes focusing on
patients in the HFO arm had improved oxygenation compared to the the key pathophysiologic mechanisms of VILI may be feasible. Another
conventional strategy. Additionally in OSCILLATE more patients in the approach on the other end of the spectrum is the use of approaches that
control arm experienced refractory hypoxemia and 11% of them crossed remove ventilation completely with the use of extracorporeal support.
over to HFO for refractory hypoxemia. Despite this, however, the death Which of these modalities will ultimately be used will depend on the results
rates due to refractory hypoxemia were not different between groups. of appropriate, high-quality clinical studies.
In patients with advanced hypoxemic and hypercapnic respiratory
failure, extracorporeal lung support (ECLS) techniques including extracor-
poreal membrane oxygenation (ECMO) have been used. These therapies KEY REFERENCES
are typically applied to ARDS patients with refractory and life-threatening
hypoxemia. The objective is to overcome severe hypoxemia and respiratory • Brower RG, et al. Higher versus lower positive end-expiratory
acidosis, while keeping the lung fully at rest. To facilitate this goal different pressures in patients with the acute respiratory distress syndrome.
venoarterial or venous-venous (with a pump) or arterial-venous (pump- N Engl J Med. 2004;351(4):327-336.
less) shunts have been used. Proponents of ECLS advocate that this method • Ferguson ND, et al. High-frequency oscillation in early acute respi-
may represent an ideal strategy of lung protection because tidal volumes ratory distress syndrome. New Engl J Med. 2013;368(9):795-805.
and PEEP can be adjusted to the optimum settings for maintaining lung • Gattinoni L, et al. Lung recruitment in patients with the acute respi-
integrity without any adverse consequence to gas exchange. Despite earlier ratory distress syndrome. N Engl J Med. 2006;354(17):1775-1786.
negative trials, 135,136 the results of the CESAR study, suggested a benefit of
• Herridge MS, et al. One-year outcomes in survivors of the acute
respiratory distress syndrome. N Engl J Med. 2003;348(8):683-693.
1.0 • Imai Y, et al. Injurious mechanical ventilation and end-organ epithe-
0.9 lial cell apoptosis and organ dysfunction in an experimental model of
acute respiratory distress syndrome. JAMA. 2003;289(16):2104-2112.
0.8 Control • Papazian L, et al. Neuromuscular blockers in early acute respira-
Probability of survival 0.6 HFOV • Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J
0.7
tory distress syndrome. N Engl J Med. 2010;363(12):1107-1116.
0.5
Med. 2013;369:2126-2136.
0.4
0.3
• Ventilation with lower tidal volumes as compared with traditional
0.2
distress syndrome. The Acute Respiratory Distress Syndrome
0.1 p = 0.004 by log-rank test tidal volumes for acute lung injury and the acute respiratory
0.0 Network. N Engl J Med. 2000;342(18):1301-1308.
0 15 30 45 60 • Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes
Days since randomization
No. at risk occult pendelluft during mechanical ventilation. Am J Respir Crit
Care Med. 2013;188:1420-1427.
HFOV 275 169 98 54 26
Control 273 181 92 54 39
FIGURE 51-4. Probability of survival from the day of randomization to day 60 in the high-
frequency oscillation and control groups. (Reproduced with permission from Ferguson ND, et al. REFERENCES
High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. February 28,
2013;368(9):795-805.) Complete references available online at www.mhprofessional.com/hall
section04.indd 448 1/23/2015 2:19:32 PM