Page 634 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 634
CHAPTER 52: Acute Lung Injury and the Acute Respiratory Distress Syndrome 453
progresses, there is extensive necrosis of type I alveolar epithelial cells. If in late-phase ARDS typically had a large dead space fraction, a high
the patient with ARDS does not recover or die during the first week, they minute ventilation requirement, progressive pulmonary hypertension,
may have a prolonged course of illness, termed late-phase ARDS. slightly improved intrapulmonary shunt that is less responsive to PEEP,
The later phase of ARDS is dominated by disordered healing. This can and a further reduction in lung compliance. 73
occur as early as 7 to 10 days after initial injury and may eventually result
in extensive pulmonary fibrosis. This has been termed the proliferative PATHOGENESIS
or fibroproliferative phase. Type II alveolar cells proliferate along alveolar
septae and the alveolar walls; fibroblasts and myofibroblasts become A number of closely interrelated pathophysiologic mechanisms and sys-
more numerous. Evidence of lung flooding is less prominent and may be tems contribute to the development of ARDS (Fig. 52-4). Dysregulated
minimal at this point. Changes in the clinical manifestations of ALI and inflammation, excess oxygen radicals, activation of coagulation and
ARDS parallel the changes in pathology. One study found that patients impaired fibrinolysis, platelet and immune cell activation, and loss of
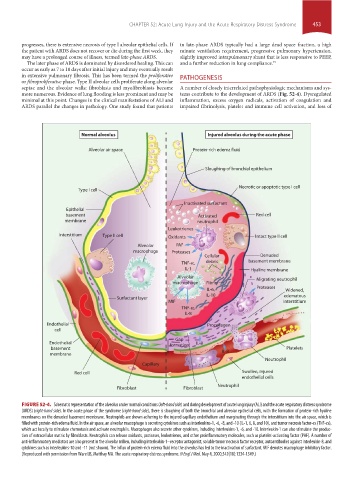
Normal alveolus Injured alveolus during the acute phase
Alveolar air space Protein-rich edema fluid
Sloughing of bronchial epithelium
Necrotic or apoptotic type I cell
Type I cell
Inactivated surfactant
Epithelial
basement Activated Red cell
membrane neutrophil
Leukotrienes
Interstitium Type II cell Oxidants Intact type II cell
Alveolar PAF
macrophage Proteases
Cellular Denuded
TNF- , debris basement membrane
IL-1 Hyaline membrane
Alveolar Migrating neutrophil
macrophage Fibrin
IL-6, Proteases Widened,
IL-10 edematous
Surfactant layer
MIF interstitium
TNF- ,
IL-8
Endothelial Procollagen
cell
Gap
Endothelial formation IL-8
basement Platelets
membrane IL-8
Neutrophil
Capillary
Red cell Swollen, injured
endothelial cells
Neutrophil
Fibroblast Fibroblast
FIGURE 52-4. Schematic representation of the alveolus under normal conditions (left-hand side) and during development of acute lung injury (ALI) and the acute respiratory distress syndrome
(ARDS) (right-hand side). In the acute phase of the syndrome (right-hand side), there is sloughing of both the bronchial and alveolar epithelial cells, with the formation of protein-rich hyaline
membranes on the denuded basement membrane. Neutrophils are shown adhering to the injured capillary endothelium and marginating through the interstitium into the air space, which is
filled with protein-rich edema fluid. In the air space, an alveolar macrophage is secreting cytokines such as interleukins-1, -6, -8, and -10 (IL-1, 6, 8, and 10), and tumor necrosis factor-α (TNF-α),
which act locally to stimulate chemotaxis and activate neutrophils. Macrophages also secrete other cytokines, including interleukins-1, -6, and -10. Interleukin-1 can also stimulate the produc-
tion of extracellular matrix by fibroblasts. Neutrophils can release oxidants, proteases, leukotrienes, and other proinflammatory molecules, such as platelet-activating factor (PAF). A number of
anti-inflammatory mediators are also present in the alveolar milieu, including interleukin-1–receptor antagonist, soluble tumor necrosis factor receptor, autoantibodies against interleukin-8, and
cytokines such as interleukins-10 and -11 (not shown). The influx of protein-rich edema fluid into the alveolus has led to the inactivation of surfactant. MIF denotes macrophage inhibitory factor.
(Reproduced with permission from Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. May 4, 2000;342(18):1334-1349.)
section04.indd 453 1/23/2015 2:19:39 PM