Page 896 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 896
CHAPTER 69: Human Immunodeficiency Virus (HIV) and AIDS in the Intensive Care Unit 627
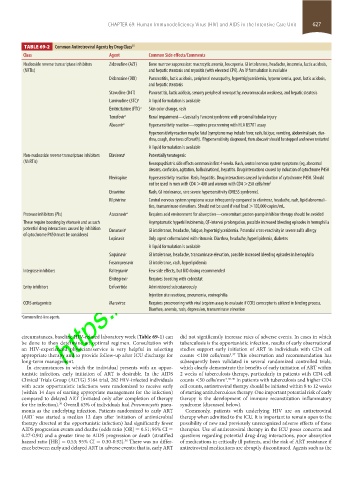
TABLE 69-2 Common Antiretroviral Agents by Drug Class 25
Class Agent Common Side effects/Comments
Nucleoside reverse transcriptase inhibitors Zidovudine (AZT) Bone marrow suppression: macrocytic anemia, leucopenia. GI intolerance, headache, insomnia, lactic acidosis,
(NRTIs) and hepatic steatosis and myositis (with elevated CPK). An IV formulation is available
Didanosine (DDI) Pancreatitis, lactic acidosis, peripheral neuropathy, hypertriglyceridemia, hyperuricemia, gout, lactic acidosis,
and hepatic steatosis
Stavudine (D4T) Pancreatitis, lactic acidosis, sensory peripheral neuropathy, neuromuscular weakness, and hepatic steatosis
Lamivudine (3TC) a A liquid formulation is available
https://kat.cr/user/tahir99/
Emtricitabine (FTC) a Skin color change, rash
Tenofovir a Renal impairment—classically Fanconi syndrome with proximal tubular injury
Abacavir a Hypersensitivity reaction—requires prescreening with HLA B5701 assay
Hypersensitivity reaction may be fatal (symptoms may include fever, rash, fatigue, vomiting, abdominal pain, diar-
rhea, cough, shortness of breath). If hypersensitivity diagnosed, then abacavir should be stopped and never restarted
A liquid formulation is available
Non-nucleoside reverse transcriptase inhibitors Efavirenz a Potentially teratogenic
(NNRTIs) Neuropsychiatric side effects common in first 4 weeks. Rash, central nervous system symptoms (eg, abnormal
dreams, confusion, agitation, hallucinations), hepatitis. Drug interactions caused by induction of cytochrome P450
Nevirapine Hypersensitivity reaction. Rash, hepatitis. Drug interactions caused by induction of cytochrome P450. Should
not be used in men with CD4 >400 and women with CD4 >250 cells/mm 3
Etravirine Rash, GI intolerance, rare severe hypersensitivity (DRESS syndrome).
Rilpivirine Central nervous system symptoms occur infrequently compared to efavirenz, headache, rash, lipid abnormali-
ties, transaminase elevations. Should not be used if viral load >100,000 copies/mL.
Protease inhibitors (PIs) Atazanavir a Requires acid environment for absorption—concomitant proton-pump inhibitor therapy should be avoided
These require boosting by ritonavir and as such Asymptomatic hyperbilirubinemia, QT-interval prolongation, possible increased bleeding episodes in hemophilia
potential drug interactions caused by inhibition Darunavir a GI intolerance, headache, fatigue, hypertriglyceridemia. Potential cross-reactivity in severe sulfa allergy
of cytochrome P450 must be considered
Lopinavir Only agent coformulated with ritonavir. Diarrhea, headache, hyperlipidemia, diabetes
A liquid formulation is available
Saquinavir GI intolerance, headache, transaminase elevation, possible increased bleeding episodes in hemophilia
Fosamprenavir GI intolerance, rash, hyperlipidemia
Integrase inhibitors Raltegravir a Few side effects, but BID dosing recommended
Elvitegravir Requires boosting with cobicistat
Entry inhibitors Enfuvirtide Administered subcutaneously
Injection site reactions, pneumonia, eosinophilia
CCR5 antagonists Maraviroc Requires prescreening with viral tropism assay to evaluate if CCR5 coreceptor is utilized in binding process.
Diarrhea, anemia, rash, depression, transaminase elevation
a Common first-line agents.
circumstances, baseline HIV-related laboratory work (Table 69-1) can did not significantly increase rates of adverse events. In cases in which
be done to then determine an optimal regimen. Consultation with tuberculosis is the opportunistic infection, results of early observational
an HIV-experienced physician/service is very helpful in selecting studies support early initiation of ART in individuals with CD4 cell
appropriate therapy and to provide follow-up after ICU discharge for counts <100 cells/mm . This observation and recommendation has
3 37
long-term management. subsequently been validated in several randomized controlled trials,
In circumstances in which the individual presents with an oppor- which clearly demonstrate the benefits of early initiation of ART within
tunistic infection, early initiation of ART is desirable. In the AIDS 2 weeks of tuberculosis therapy, particularly in patients with CD4 cell
Clinical Trials Group (ACTG) 5164 trial, 282 HIV-infected individuals counts <50 cells/mm . In patients with tuberculosis and higher CD4
3 38-40
with acute opportunistic infections were randomized to receive early cell counts, antiretroviral therapy should be initiated within 8 to 12 weeks
(within 14 days of starting appropriate management for the infection) of starting antituberculous therapy. One important potential risk of early
compared to delayed ART (initiated only after completion of therapy therapy is the development of immune reconstitution inflammatory
for the infection). Overall 63% of individuals had Pneumocystis pneu- syndrome (discussed below).
36
monia as the underlying infection. Patients randomized to early ART Commonly, patients with underlying HIV are on antiretroviral
(ART was started a median 12 days after initiation of antimicrobial therapy when admitted to the ICU. It is important to remain open to the
therapy directed at the opportunistic infection) had significantly fewer possibility of new and previously unrecognized adverse effects of these
AIDS progression events and deaths (odds ratio [OR] = 0.51; 95% CI = therapies. Use of antiretroviral therapy in the ICU poses concerns and
0.27-0.94) and a greater time to AIDS progression or death (stratified questions regarding potential drug-drug interactions, poor absorption
hazard ratio [HR] = 0.53; 95% CI = 0.30-0.92). There was no differ- of medications in critically ill patients, and the risk of ART resistance if
36
ence between early and delayed ART in adverse events; that is, early ART antiretroviral medications are abruptly discontinued. Agents such as the
section05_c61-73.indd 627 1/23/2015 12:48:14 PM