Page 138 - Cardiac Nursing
P. 138
009
009
9/2
9/2
8:2
8:2
0
0
9/0
31.
qxd
1-1
31.
0
9/0
qxd
0
4 A
14
14
e 1
e 1
ara
ara
Apt
Apt
g
M
P
4 A
M
a
g
P
a
0-c
0-c
K34
05_
L L LWB
05_
p11
p11
1-1
K34
LWB
LWBK340-c05_p111-131.qxd 09/09/2009 08:24 AM Page 114 Aptara
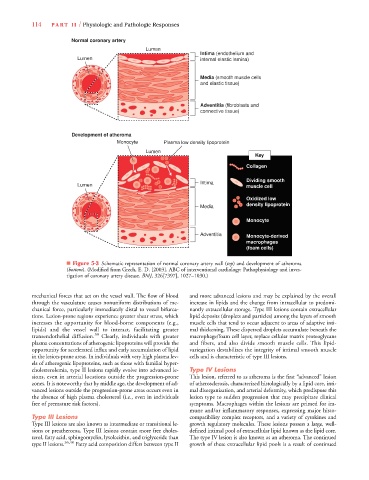
114 PA R T I I / Physiologic and Pathologic Responses
Normal coronary artery
Lumen
Intima (endothelium and
Lumen internal elastic lamina)
Media (smooth muscle cells
and elastic tissue)
Adventitia (fibroblasts and
connective tissue)
Development of atheroma
Monocyte Plasma low density lipoprotein
Lumen
Key
Collagen
Dividing smooth
Intima
Lumen muscle cell
Oxidized low
density lipoprotein
Media
Monocyte
Adventitia Monocyte-derived
macrophages
(foam cells)
■ Figure 5-3 Schematic representation of normal coronary artery wall (top) and development of atheroma
(bottom). (Modified from Grech, E. D. [2003]. ABC of interventional cardiology: Pathophysiology and inves-
tigation of coronary artery disease. BMJ, 326[7397], 1027–1030.)
mechanical forces that act on the vessel wall. The flow of blood and more advanced lesions and may be explained by the overall
through the vasculature causes nonuniform distributions of me- increase in lipids and the change from intracellular to predomi-
chanical force, particularly immediately distal to vessel bifurca- nantly extracellular storage. Type III lesions contain extracellular
tions. Lesion-prone regions experience greater shear stress, which lipid deposits (droplets and particles) among the layers of smooth
increases the opportunity for blood-borne components (e.g., muscle cells that tend to occur adjacent to areas of adaptive inti-
lipids) and the vessel wall to interact, facilitating greater mal thickening. These dispersed droplets accumulate beneath the
transendothelial diffusion. 39 Clearly, individuals with greater macrophage/foam cell layer, replace cellular matrix proteoglycans
plasma concentrations of atherogenic lipoproteins will provide the and fibers, and also divide smooth muscle cells. This lipid-
opportunity for accelerated influx and early accumulation of lipid variegation destabilizes the integrity of intimal smooth muscle
in the lesion-prone areas. In individuals with very high plasma lev- cells and is characteristic of type III lesions.
els of atherogenic lipoproteins, such as those with familial hyper-
cholesterolemia, type II lesions rapidly evolve into advanced le- Type IV Lesions
sions, even in arterial locations outside the progression-prone This lesion, referred to as atheroma is the first “advanced” lesion
zones. It is noteworthy that by middle age, the development of ad- of atherosclerosis, characterized histologically by a lipid core, inti-
vanced lesions outside the progression-prone areas occurs even in mal disorganization, and arterial deformity, which predispose this
the absence of high plasma cholesterol (i.e., even in individuals lesion type to sudden progression that may precipitate clinical
free of premature risk factors). symptoms. Macrophages within the lesions are primed for im-
mune and/or inflammatory responses, expressing major histo-
Type III Lesions compatibility complex receptors, and a variety of cytokines and
Type III lesions are also known as intermediate or transitional le- growth regulatory molecules. These lesions possess a large, well-
sions or preatheroma. Type III lesions contain more free choles- defined intimal pool of extracellular lipid known as the lipid core.
terol, fatty acid, sphingomyelin, lysolecithin, and triglyceride than The type IV lesion is also known as an atheroma. The continued
type II lesions. 36,40 Fatty acid composition differs between type II growth of these extracellular lipid pools is a result of continued