Page 566 - Cardiac Nursing
P. 566
009
0
9/2
009
0
9 A
9 A
8:2
8:2
qxd
qxd
54.
54.
0
9/0
9/2
0
9/0
42
42
e 5
e 5
42
ara
ara
Apt
Apt
P
P
M
M
P
g
g
a
a
0-c
K34
0-c
23_
23_
LWB
LWBK340-c23_23_p537-554.qxd 09/09/2009 08:29 AM Page 542 Aptara
L L LWB
K34
K34
p53
p53
7-5
54.
7-5
542 PA R T I V / Pathophysiology and Management of Heart Disease
■ Figure 23-6 The BX Velocity Coronary Stent used for delivery
of Sirolimus: The CYPHERR ™ Sirolimus-Eluding Stent. (Courtesy of
Cordis, A Johnson & Johnson Company, Miami Lakes, FL.)
coronary vessel leading to vascular complications. The clinical
success of this technology depends on the complex interaction be-
tween the stent, coating matrix, drug, and vessel wall. 34
Sirolimus-Eluting Stent (SES)
The first DES approved for use in PCI by the FDA in 2003 was
the CYPHERR TM sirolimus-eluting stent (Fig. 23-6). The CYPHER
stent has a stainless steel stent platform, the BX Velocity, covered ™
with a thin polymer coating containing the drug sirolimus. The ■ Figure 23-8 The TAXUS Stent. The Express 2 Stent is used as
slow-release formulation inhibits cell proliferation by targeting the platform for the Paclitaxel-Eluting Stent. A dime is placed next to
the device to represent size. (Courtesy of Boston Scientific Corpora-
smooth muscle cells, while simultaneously reducing inflamma- tion, Maple Grove, MN.)
tory cytokine production and resultant vessel wall inflamma-
tion. 35
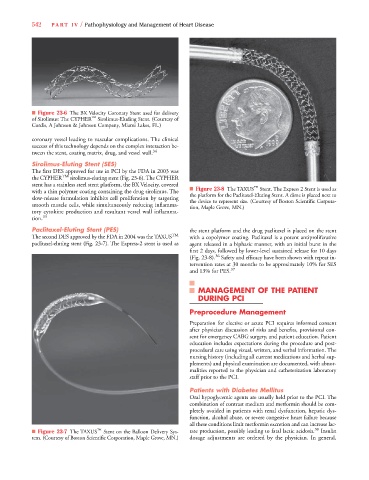
Paclitaxel-Eluting Stent (PES) the stent platform and the drug paclitaxel is placed on the stent
The second DES approved by the FDA in 2004 was the TAXUS TM with a copolymer coating. Paclitaxel is a potent antiproliferative
paclitaxel-eluting stent (Fig. 23-7). The Express-2 stent is used as agent released in a biphasic manner, with an initial burst in the
first 2 days, followed by lower-level sustained release for 10 days
(Fig. 23-8). 36 Safety and efficacy have been shown with repeat in-
tervention rates at 30 months to be approximately 10% for SES
and 13% for PES. 37
MANAGEMENT OF THE PATIENT
DURING PCI
Preprocedure Management
Preparation for elective or acute PCI requires informed consent
after physician discussion of risks and benefits, provisional con-
sent for emergency CABG surgery, and patient education. Patient
education includes expectations during the procedure and post-
procedural care using visual, written, and verbal information. The
nursing history (including all current medications and herbal sup-
plements) and physical examination are documented, with abnor-
malities reported to the physician and catheterization laboratory
staff prior to the PCI.
Patients with Diabetes Mellitus
Oral hypoglycemic agents are usually held prior to the PCI. The
combination of contrast medium and metformin should be com-
pletely avoided in patients with renal dysfunction, hepatic dys-
function, alcohol abuse, or severe congestive heart failure because
all these conditions limit metformin excretion and can increase lac-
■ Figure 23-7 The TAXUS ™ Stent on the Balloon Delivery Sys- tate production, possibly leading to fatal lactic acidosis. 38 Insulin
tem. (Courtesy of Boston Scientific Corporation, Maple Grove, MN.) dosage adjustments are ordered by the physician. In general,