Page 812 - Cardiac Nursing
P. 812
LWBK340-c34_p783-798.qxd 30/06/2009 12:07 AM Page 788 Aptara
788 PA R T V / Health Promotion and Disease Prevention
the initial quit date, leading many to conclude that there is no safe
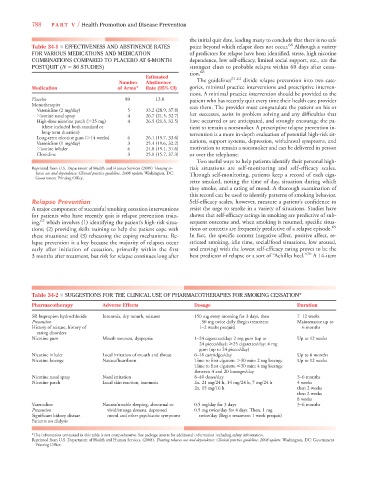
Table 34-1 ■ EFFECTIVENESS AND ABSTINENCE RATES point beyond which relapse does not occur. 68 Although a variety
FOR VARIOUS MEDICATIONS AND MEDICATION of predictors for relapse have been identified, stress, high nicotine
COMBINATIONS COMPARED TO PLACEBO AT 6-MONTH dependence, low self-efficacy, limited social support, etc., are the
(
(
POSTQUIT (N 86 STUDIES) strongest clues to probable relapse within 60 days after cessa-
tion. 68
Estimated 61,62
Number Abstinence The guidelines divide relapse prevention into two cate-
Medication of Arms* Rate (95% CI) gories, minimal practice interventions and prescriptive interven-
tions. A minimal practice intervention should be provided to the
Placebo 80 13.8 patient who has recently quit every time their health care provider
Monotherapies
Varenicline (2 mg/day) 5 33.2 (28.9, 37.8) sees them. The provider must congratulate the patient on his or
Nicotine nasal spray 4 26.7 (21.5, 32.7) her successes, assist in problem solving and any difficulties that
High-dose nicotine patch ( 25 mg) 4 26.5 (21.3, 32.5) have occurred or are anticipated, and strongly encourage the pa-
(these included both standard or tient to remain a nonsmoker. A prescriptive relapse prevention in-
long-term duration) tervention is a more in-depth evaluation of potential high-risk sit-
Long-term nicotine gum ( 14 weeks) 6 26.1 (19.7, 33.6)
Varenicline (1 mg/day) 3 25.4 (19.6, 32.2) uations, support systems, depression, withdrawal symptoms, and
Nicotine inhaler 6 24.8 (19.1, 31.6) motivation to remain a nonsmoker and can be delivered in person
Clonidine 3 25.0 (15.7, 37.3) or over the telephone.
Two useful ways to help patients identify their personal high-
Reprinted from U.S. Department of Health and Human Services (2008) Treating to- risk situations are self-monitoring and self-efficacy scales.
bacco use and dependence: Clinical practice guideline, 2008 update. Washington, DC: Through self-monitoring, patients keep a record of each ciga-
Government Printing Office.
rette smoked, noting the time of day, situation during which
they smoke, and a rating of mood. A thorough examination of
this record can be used to identify patterns of smoking behavior.
Relapse Prevention Self-efficacy scales, however, measure a patient’s confidence to
A major component of successful smoking cessation interventions resist the urge to smoke in a variety of situations. Studies have
for patients who have recently quit is relapse prevention train- shown that self-efficacy ratings in smoking are predictive of sub-
ing, 67 which involves (1) identifying the patient’s high-risk situa- sequent outcome and, when smoking is resumed, specific situa-
tions; (2) providing skills training to help the patient cope with tions or contexts are frequently predictive of a relapse episode. 69
these situations; and (3) rehearsing the coping mechanisms. Re- In fact, the specific context (negative affect, positive affect, re-
lapse prevention is a key because the majority of relapses occur stricted smoking, idle time, social/food situations, low arousal,
early after initiation of cessation, primarily within the first and craving) with the lowest self-efficacy rating proves to be the
3 months after treatment, but risk for relapse continues long after best predictor of relapse or a sort of “Achilles heel.” 70 A 14-item
Table 34-2 ■ SUGGESTIONS FOR THE CLINICAL USE OF PHARMACOTHERAPIES FOR SMOKING CESSATION*
Pharmacotherapy Adverse Effects Dosage Duration
SR bupropion hydrochloride Insomnia, dry mouth, seizures 150 mg every morning for 3 days, then 7–12 weeks
Precaution 150 mg twice daily (begin treatment Maintenance up to
History of seizure, history of 1–2 weeks prequit) 6 months
eating disorders
Nicotine gum Mouth soreness, dyspepsia 1–24 cigarettes/day: 2 mg gum (up to Up to 12 weeks
24 pieces/day); 25 cigarettes/day: 4 mg
gum (up to 24 pieces/day)
Nicotine inhaler Local irritation of mouth and throat 6–16 cartridges/day Up to 6 months
Nicotine lozenge Nausea/heartburn Time to first cigarette 30 min: 2 mg lozenge Up to 12 weeks
Time to first cigarette 30 min: 4 mg lozenge
Between 4 and 20 lozenges/day
Nicotine nasal spray Nasal irritation 8–40 doses/day 3–6 months
Nicotine patch Local skin reaction, insomnia Ex. 21 mg/24 h, 14 mg/24 h, 7 mg/24 h 4 weeks
Ex. 15 mg/16 h then 2 weeks
then 2 weeks
8 weeks
Varenicline Nausea/trouble sleeping, abnormal or 0.5 mg/day for 3 days 3–6 months
Precaution vivid/strange dreams, depressed 0.5 mg twice/day for 4 days. Then, 1 mg
Significant kidney disease mood and other psychiatric symptoms twice/day (Begin treatment 1 week prequit)
Patients on dialysis
*The information contained in this table is not comprehensive. See package inserts for additional information including safety information.
Reprinted from U.S. Department of Health and Human Services. (2008). Treating tobacco use and dependence: Clinical practice guideline, 2008 update. Washington, DC: Government
Printing Office.