Page 112 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 112
Chapter 8 Pharmacogenomics and Hematologic Diseases 83
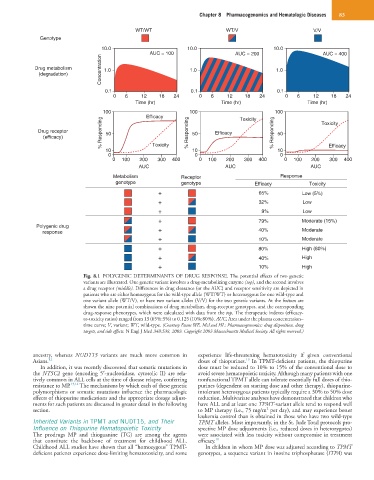
WT/WT WT/V V/V
Genotype
10.0 10.0 10.0
AUC = 100 AUC = 200 AUC = 400
Concentration 1.0 1.0 1.0
Drug metabolism
(degradation)
0.1 0.1 0.1
0 6 12 18 24 0 6 12 18 24 0 6 12 18 24
Time (hr) Time (hr) Time (hr)
100 100 100
Efficacy Toxicity Toxicity
% Responding 50 % Responding 50 Efficacy % Responding 50
Drug receptor
(efficacy)
10 Toxicity 10 10 Efficacy
0 0 0
0 100 200 300 400 0 100 200 300 400 0 100 200 300 400
AUC AUC AUC
Metabolism Receptor Response
genotype genotype Efficacy Toxicity
+ 65% Low (5%)
+ 32% Low
+ 9% Low
+ 79% Moderate (15%)
Polygenic drug
response + 40% Moderate
+ 10% Moderate
+ 80% High (80%)
+ 40% High
+ 10% High
Fig. 8.1 POLYGENIC DETERMINANTS OF DRUG RESPONSE. The potential effects of two genetic
variants are illustrated. One genetic variant involves a drug-metabolizing enzyme (top), and the second involves
a drug receptor (middle). Differences in drug clearance (or the AUC) and receptor sensitivity are depicted in
patients who are either homozygous for the wild-type allele (WT/WT) or heterozygous for one wild-type and
one variant allele (WT/V), or have two variant alleles (V/V) for the two genetic variants. At the bottom are
shown the nine potential combinations of drug metabolism, drug-receptor genotypes, and the corresponding
drug-response phenotypes, which were calculated with data from the top. The therapeutic indexes (efficacy-
to-toxicity ratios) ranged from 13 (65%:5%) to 0.125 (10%:80%). AUC, Area under the plasma concentration–
time curve; V, variant; WT, wild-type. (Courtesy Evans WE, McLeod HL: Pharmacogenomics: drug disposition, drug
targets, and side effects. N Engl J Med 348:538, 2003. Copyright 2003 Massachusetts Medical Society. All rights reserved.)
ancestry, whereas NUDT15 variants are much more common in experience life-threatening hematotoxicity if given conventional
10
Asians. 12 doses of thiopurines. In TPMT-deficient patients, the thiopurine
In addition, it was recently discovered that somatic mutations in dose must be reduced to 10% to 15% of the conventional dose to
the NT5C2 gene (encoding 5′-nucleotidase, cytosolic II) are rela- avoid severe hematopoietic toxicity. Although many patients with one
tively common in ALL cells at the time of disease relapse, conferring nonfunctional TPMT allele can tolerate essentially full doses of thio-
resistance to MP. 13,14 The mechanisms by which each of these genetic purines (dependent on starting dose and other therapy), thiopurine-
polymorphisms or somatic mutations influence the pharmacologic intolerant heterozygous patients typically require a 30% to 50% dose
effects of thiopurine medications and the appropriate dosage adjust- reduction. Multivariate analyses have demonstrated that children who
ments for such patients are discussed in greater detail in the following have ALL and at least one TPMT-variant allele tend to respond well
2
section. to MP therapy (i.e., 75 mg/m per day), and may experience better
leukemia control than is obtained in those who have two wild-type
Inherited Variants in TPMT and NUDT15, and Their TPMT alleles. Most importantly, in the St. Jude Total protocols pro-
Influence on Thiopurine Hematopoietic Toxicity spective MP dose adjustments (i.e., reduced doses in heterozygotes)
The prodrugs MP and thioguanine (TG) are among the agents were associated with less toxicity without compromise in treatment
that constitute the backbone of treatment for childhood ALL. efficacy. 10
Childhood ALL studies have shown that all “homozygous” TPMT- In children in whom MP dose was adjusted according to TPMT
deficient patients experience dose-limiting hematotoxicity, and some genotypes, a sequence variant in inosine triphosphatase (ITPA) was