Page 113 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 113
84 Part I Molecular and Cellular Basis of Hematology
Genotype/Phenotype Drug dose Systemic exposure Toxicity
TPMT alleles Conventional dosing
*1 500 1
ATG 5000 Deficient
*2 4000 0.8
0.6
ATG 238>C 250 TGN (pmol/8 × 10 8 RBC) 3000 Cumulative incidence 0.4 Heterozygote
2000
TPMT deficiency *3A ATG 460G>A 719A>C 0 v/v wt/v wt/wt 1000 Deficient Wild-type 0.2 0 0 0.5 1.5 Wild-type
0
2 2.5
*3C
Heterozygote
ATG 719A>G TPMT phenotype 1 Years
10 Individualized dosing
wt/wt
8 500 5000 0.8 1
Percent 6 4 *2, *3A, *3C MP mg/m 2 /wt 250 TGN (pmol/8 × 10 8 RBC) 4000 Cumulative incidence 0.4
0.6
3000
2 v/v wt/v 2000 0.2
1000
0 0 0 0
0 10 20 30 v/v wt/v wt/wt Deficient Wild-type 0 0.5 1 1.5 2 2.5
TPMT actvity Heterozygote Years
TPMT phenotype
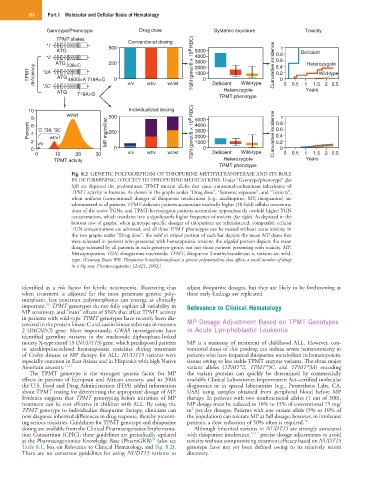
Fig. 8.2 GENETIC POLYMORPHISM OF THIOPURINE METHYLTRANSFERASE AND ITS ROLE
IN DETERMINING TOXICITY TO THIOPURINE MEDICATIONS. Under “Genotype/phenotype” (far
left) are depicted the predominant TPMT mutant alleles that cause autosomal-codominant inheritance of
TPMT activity in humans. As shown in the graphs under “Drug dose”, “Systemic exposure”, and “Toxicity”,
when uniform (conventional) dosages of thiopurine medications (e.g., azathioprine, MP, thioguanine) are
administered to all patients, TPMT-deficient patients accumulate markedly higher (10-fold) cellular concentra-
tions of the active TGNs, and TPMT-heterozygous patients accumulate approximately twofold higher TGN
concentrations, which translates into a significantly higher frequency of toxicity (far right). As depicted in the
bottom row of graphs, when genotype-specific dosages of thiopurines are administered, comparable cellular
TGN concentrations are achieved, and all three TPMT phenotypes can be treated without acute toxicity. In
the two graphs under “Drug dose”, the solid or striped portion of each bar depicts the mean MP doses that
were tolerated in patients who presented with hematopoietic toxicity; the stippled portion depicts the mean
dosage tolerated by all patients in each genotype group, not just those patients presenting with toxicity. MP,
Mercaptopurine; TGN, thioguanine nucleotide; TPMT, thiopurine S-methyltransferase; v, variant; wt, wild-
type. (Courtesy Evans WE: Thiopurine S-methyltransferase: a genetic polymorphism that affects a small number of drugs
in a big way. Pharmacogenetics 12:421, 2002.)
identified as a risk factor for febrile neutropenia, illustrating that adjust thiopurine dosages, but they are likely to be forthcoming as
when treatment is adjusted for the most penetrant genetic poly- these early findings are replicated.
morphism, less penetrant polymorphisms can emerge as clinically
10
important. TPMT genotypes do not fully explain all variability in Relevance to Clinical Hematology
MP sensitivity, and “trans” effects of SNPs that affect TPMT activity
in patients with wild-type TPMT genotypes have recently been dis-
covered in the protein kinase C and casein kinase substrate in neurons MP Dosage Adjustment Based on TPMT Genotypes
2 (PACSIN2) gene. More importantly, GWAS investigations have in Acute Lymphoblastic Leukemia
identified germline variants in the nucleoside diphosphate-linked
moiety X-type motif 15 (NUDT15) gene, which predisposed patients MP is a mainstay of treatment of childhood ALL. However, con-
to azathioprine-related hematopoietic toxicities during treatment ventional doses of this prodrug can induce severe hematotoxicity in
of Crohn disease or MP therapy for ALL: NUDT15 variants were patients who have impaired thiopurine metabolism in hematopoietic
especially common in East Asians and in Hispanics with high Native tissues owing to less stable TPMT enzyme variants. The three major
American ancestry. 11,12 variant alleles (TPMT*2, TPMT*3C, and TPMT*3A) encoding
The TPMT genotype is the strongest genetic factor for MP the variant proteins can quickly be determined by commercially
effects in patients of European and African ancestry, and in 2004 available Clinical Laboratories Improvement Act–certified molecular
the U.S. Food and Drug Administration (FDA) added information diagnostics or in special laboratories (e.g., Prometheus Labs, CA,
about TPMT testing for determining the appropriate dosage of MP. USA) using samples obtained from peripheral blood before MP
Evidence suggests that TPMT genotyping before initiation of MP therapy. In patients with two nonfunctional alleles (1 out of 300),
treatment can be cost effective in children with ALL. By using the MP dosage must be reduced to 10% to 15% of conventional 75 mg/
2
TPMT genotype to individualize thiopurine therapy, clinicians can m per day dosages. Patients with one variant allele (5% to 10% of
now diagnose inherited differences in drug response, thereby prevent- the population) can tolerate MP at full dosage; however, in intolerant
ing serious toxicities. Guidelines for TPMT genotype and thiopurine patients, a dose reduction of 50% often is required. 10
dosing are available from the Clinical Pharmacogenetics Implementa- Although inherited variants in NUDT15 are strongly associated
tion Consortium (CPIC); these guidelines are periodically updated with thiopurine intolerance, 11,12 precise dosage adjustments to avoid
10
at the Pharmacogenomics Knowledge Base (PharmGKB) (also see toxicity without compromising treatment efficacy based on NUDT15
Table 8.1, box on Relevance to Clinical Hematology, and Fig. 8.2). genotype have not yet been defined owing to its relatively recent
There are no consensus guidelines for using NUDT15 variants to discovery.