Page 1131 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1131
Chapter 63 Myelodysplastic Syndromes and Myeloproliferative Neoplasms in Children 997
100
Treatment Overview for Children With MDS
90
A multitude of agents have been studied for the treatment of myelodys- 80
plastic syndromes (MDS) in adults, but only rarely in children. These 70
include low-dose chemotherapy (cytosine arabinoside, melphalan, 60
hydroxyurea, etoposide, topotecan, 6-mercaptopurine, and busulfan),
hormones (glucocorticoids and androgens), differentiating agents Probability of DFS, % 50 Matched, 57% (35–74)
(13-cis-retinoic acid, all-trans retinoic acid), hematopoietic growth 40
factors (granulocyte-macrophage colony-forming factor, granulocyte 30
colony-forming factor, and erythropoietin), demethylating agents Mismatched, 33% (23–43)
(decitabine, 5-azacytidine), proteosome inhibitors, antiangiogenic 20
agents, and arsenic. 60–75 This has resulted in three drugs (lenalidomide, 10 P = .003
azacitidine, and decitabine) that are now approved by the US Food 0
and Drug Administration for the treatment of MDS in adults. However, 0 1 2 3 4 5 6 7 8
as there are currently no safety or efficacy data to support the use of
these agents in children with MDS, these drugs are not approved for A Years
MDS in children.
In addition, a large number of agents are being tested in clinical trials
in the broad categories of kinase inhibitors, deacetylase inhibitors and 100
DNA methyltransferase inhibitors, altered cell metabolism, cytotoxics, 90
cell cycle inhibitors, immunomodulators and immunosuppressive 80
agents, apoptosis modulators, and others. 76 70
The difficulty in assessing the safety and efficacy of new agents in
children with MDS is illustrated by the Children’s Oncology Group’s 60 RC, 51% (34–65)
77
recent prospective study of amifostine. This prospective Phase II Probability of DFS, % 50
cooperative group study was unable to be completed because of lack 40 RAEB, 35% (22–48)
of accrual. As a result, the safety and efficacy of amifostine in children
with MDS remains uncertain. Despite a lack of successful prospective 30 RAEB-t, 29% (11–51)
clinical trials in children with MDS, there are limited retrospective 20
data on the use of the hypomethylating agent azacytidine in children 10 P = .04
with newly diagnosed MDS, and in the palliative setting, children with 0
relapsed MDS. These children had RCC, advanced and secondary
MDS, and prior to allogeneic hematopoietic cell transplantation 0 1 2 3 4 5 6 7 8
78
(HCT). These retrospective reviews suggest that azacytidine is toler- B Years
79
able and responses are possible, although the safety and efficacy in
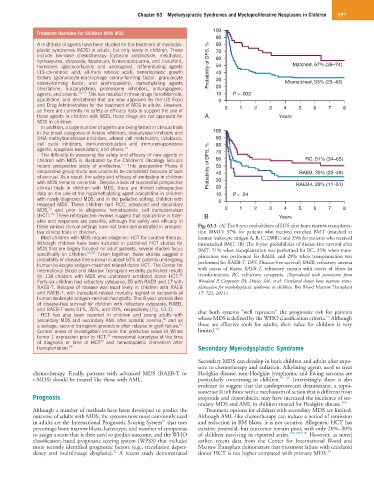
these various clinical settings have not been demonstrated in prospec- Fig. 63.3 (A) The 8-year probabilities of DFS after bone marrow transplanta-
tive clinical trials in children. tion (BMT): 57% for patients who received matched BMT (matched at
Most children with MDS require allogeneic HCT for curative therapy. human leukocyte antigen A, B, C, DRB1) and 33% for patients who received
Although children have been included in published HCT studies for mismatched BMT. (B) The 8-year probabilities of disease-free survival after
MDS that are largely focused on adult patients, several studies focus BMT: 51% when transplantation was performed for RC, 35% when trans-
specifically on children. 80–85 Taken together, these studies suggest a plantation was performed for RAEB, and 29% when transplantation was
probability of disease-free survival in about 50% of patients undergoing performed for RAEB-T. DFS, Disease-free survival; RAEB, refractory anemia
human leukocyte antigen–matched related donor HCT. The Center for
International Blood and Marrow Transplant recently published results with excess of blasts; RAEB-T, refractory anemia with excess of blasts in
for 118 children with MDS who underwent unrelated donor HCT. transformation; RC, refractory cytopenia. (Reproduced with permission from
84
Forty-six children had refractory cytopenia, 55 with RAEB and 17 with Woodard P, Carpenter PA, Davies SM, et al: Unrelated donor bone marrow trans-
RAEB-T. Relapse of disease was most likely in children with RAEB plantation for myelodysplastic syndrome in children. Bio Blood Marrow Transplant
and RAEB-T, with transplant-related mortality highest in recipients of 17: 723, 2011.)
human leukocyte antigen–mismatched grafts. The 8-year probabilities
of disease-free survival for children with refractory cytopenia, RAEB,
and RAEB-T were 51%, 35%, and 29%, respectively (Fig. 63.3). that both systems “well represent” the prognostic risk for patients
HCT has also been reported in children and young adults with 93
86
secondary MDS and secondary AML after aplastic anemia, and as whose MDS is defined by the WHO classification criteria. Although
87
a salvage, second transplant procedure after relapse or graft failure. these are effective tools for adults, their value for children is very
94
Current areas of investigation include the predictive value of Wilms limited.
tumor 1 expression prior to HCT, monosomal karyotype at the time
88
of diagnosis or time of HCT and hematopoietic chimerism after
89
transplantation. 90 Secondary Myelodysplastic Syndrome
Secondary MDS can develop in both children and adults after expo-
sure to chemotherapy and radiation. Alkylating agents used to treat
chemotherapy. Finally, patients with advanced MDS (RAEB-T or Hodgkin disease, non-Hodgkin lymphoma, and Ewing sarcoma are
t-MDS) should be treated like those with AML. particularly concerning in children. 95–107 Interestingly, there is also
evidence to suggest that the cardioprotectant dexrazoxane, a topoi-
somerase II inhibitor with a mechanism of action that is different from
Prognosis etoposide and doxorubicin, may have increased the incidence of sec-
ondary MDS and AML in children treated for Hodgkin disease. 108
Although a number of methods have been developed to predict the Treatment options for children with secondary MDS are limited.
outcome of adults with MDS, the systems now most commonly used Although AML-like chemotherapy can induce a period of remission
91
in adults are the International Prognostic Scoring System that uses and reduction in BM blasts, it is not curative. Allogeneic HCT has
percentage bone marrow blasts, karyotype, and number of cytopenias curative potential, but outcomes remain poor, with only 20%–30%
to assign a score that is then used to predict outcome, and the WHO of children surviving in reported series. 106,109,110 However, as noted
classification-based prognostic scoring system (WPSS) that includes earlier, recent data from the Center for International Blood and
more recently identified prognostic factors (e.g., transfusion depen- Marrow Transplant demonstrate that treatment failure with unrelated
92
dency and multilineage dysplasia). A recent study demonstrated donor HCT is not higher compared with primary MDS. 84