Page 1134 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1134
1000 Part VII Hematologic Malignancies
C
A E
B D F
G H I J K L
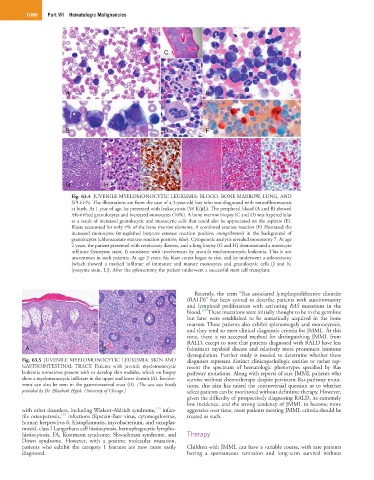
Fig. 63.4 JUVENILE MYELOMONOCYTIC LEUKEMIA: BLOOD, BONE MARROW, LUNG, AND
SPLEEN. The illustrations are from the case of a 3-year-old boy who was diagnosed with neurofibromatosis
at birth. At 1 year of age, he presented with leukocytosis (58 K/µL). The peripheral blood (A and B) showed
left-shifted granulocytes and increased monocytes (16%). A bone marrow biopsy (C and D) was hypercellular
as a result of increased granulocytic and monocytic cells that could also be appreciated on the aspirate (E).
Blasts accounted for only 4% of the bone marrow elements. A combined esterase reaction (F) illustrated the
increased monocytes (α-naphthol butyrate esterase reaction positive; orange/brown) in the background of
granulocytes (chloroacetate esterase reaction positive; blue). Cytogenetic analysis revealed monosomy 7. At age
2 years, the patient presented with respiratory distress, and a lung biopsy (G and H) demonstrated a monocyte
infiltrate (lysozyme stain, I) consistent with involvement by juvenile myelomonocytic leukemia. This is not
uncommon in such patients. At age 3 years, his blast count began to rise, and he underwent a splenectomy
(which showed a marked infiltrate of immature and mature monocytes and granulocytic cells [J and K;
lysozyme stain, L]). After the splenectomy the patient underwent a successful stem cell transplant.
Recently, the term “Ras associated lymphoproliferative disorder
(RALD)” has been coined to describe patients with autoimmunity
and lymphoid proliferation with activating RAS mutations in the
175
blood. These mutations were initially thought to be in the germline
but later were established to be somatically acquired in the bone
L marrow. These patients also exhibit splenomegaly and monocytosis,
and they tend to meet clinical diagnostic criteria for JMML. At this
time, there is no accepted method for distinguishing JMML from
RALD, except to note that patients diagnosed with RALD have less
A B fulminant myeloid disease and relatively more prominent immune
dysregulation. Further study is needed to determine whether these
Fig. 63.5 JUVENILE MYELOMONOCYTIC LEUKEMIA: SKIN AND diagnoses represent distinct clinicopathologic entities or rather rep-
GASTROINTESTINAL TRACT. Patients with juvenile myelomonocytic resent the spectrum of hematologic phenotypes specified by Ras
leukemia sometimes present with or develop skin nodules, which on biopsy pathway mutations. Along with reports of rare JMML patients who
show a myelomonocytic infiltrate in the upper and lower dermis (A). Involve- survive without chemotherapy despite persistent Ras pathway muta-
ment can also be seen in the gastrointestinal tract (B). (The case was kindly tions, this idea has raised the controversial question as to whether
provided by Dr. Elizabeth Hyjek, University of Chicago.) select patients can be monitored without definitive therapy. However,
given the difficulty of prospectively diagnosing RALD, its extremely
low incidence, and the strong tendency of JMML to become more
173
with other disorders, including Wiskott-Aldrich syndrome, infan- aggressive over time, most patients meeting JMML criteria should be
174
tile osteopetrosis, infections (Epstein-Barr virus, cytomegalovirus, treated as such.
human herpesvirus 6, histoplasmosis, mycobacterium, and toxoplas-
mosis), class I Langerhans cell histiocytosis, hemophagocytic lympho-
histiocytosis, FA, Kostmann syndrome, Shwachman syndrome, and Therapy
Down syndrome. However, with a positive molecular mutation,
patients who exhibit the category 1 features are now more easily Children with JMML can have a variable course, with rare patients
diagnosed. having a spontaneous remission and long-term survival without