Page 1220 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1220
1066 Part VII Hematologic Malignancies
According to STIM (A-STIM) study evaluated whether the loss of A
MMR, defined as <0.1% BCR-ABL (IS), was a safe and clinically 1.0
relevant measure for defining molecular relapse after imatinib discon- Chronic phase (n = 303)
tinuation. Eighty patients with CP-CML in prolonged CMR were
studied. Cumulative MMR loss was 36% at 24 months, whereas 0.8
probability of losing CMR was higher. Fluctuation of BCR-ABL
transcript levels below the MMR threshold was observed in 31% of
patients. Treatment-free remission was estimated as 61% at 36 0.6 Accelerated phase/blast
crises remission (n = 359)
months. These results suggest that loss of MMR is a practical and
safe criterion for restarting therapy after treatment discontinuation Probability of survival
in CML patients with prolonged CMR. 0.4
Management of Pregnancy in CML Patients 0.2 Blast crisis (n = 20)
Imatinib therapy has been associated with a constellation of rare
congenital malformations and spontaneous abortions. All couples 0.0
should be counselled on the risks associated with pregnancy whilst 0 2 4 6 8 10
receiving TKI therapy. At the time of diagnosis, fertility preservation Years after transplant
should be discussed with both male and female patients of childbear-
ing potential. Patients should be counselled regarding fertility options B
such as semen cryopreservation, oocyte retrieval and storage, and 1.0
embryo cryopreservation because of potential deleterious effects of
TKIs on gonadal function and fertility. Pregnancy in CML presents
specific management challenges, and requires a multidisciplinary 0.8 Chronic phase (n = 168)
approach with close collaboration with the obstetricians. Manage-
ment of patients who become pregnant whilst receiving TKI therapy
requires balancing risks to the fetus of continuing therapy versus risks 0.6
to the patient from treatment interruption and loss of disease control. Accelerated phase/blast
Patients presenting with CP CML during pregnancy could safely Probability of survival crises remission (n = 49)
continue their pregnancy to term and be successfully managed with 0.4
leukapheresis during the first and subsequent trimesters, and intro-
duction of IFN in the second trimester onwards if needed. Patients Blast crisis (n = 10)
presenting in advanced phase disease should consider elective termi- 0.2
nation of pregnancy in order to commence induction chemotherapy
and/or a TKI. 25
0.0
0 2 4 6 8 10
Allogeneic Hematopoietic Cell Transplantation Years after transplant
Allogeneic HCT is a well-established treatment for CML, associated Fig. 67.8 Probability of survival following (A) related donor transplantation
with the possibility of long-term disease-free survival, but has largely and (B) unrelated donor transplantation for chronic myeloid leukemia,
been replaced as an initial curative strategy as a result of the success chronic phase, accelerated phase, and blast crisis performed after 1992, at the
of TKI treatment of CML. Fred Hutchinson Cancer Research Center, Seattle.
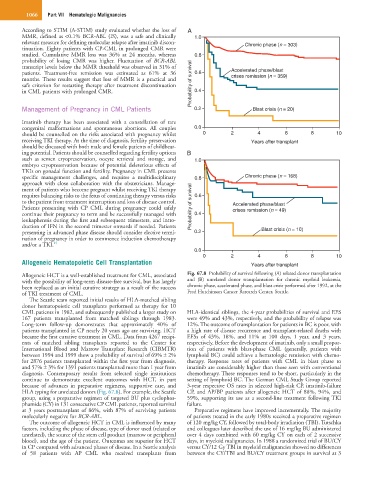
The Seattle team reported initial results of HLA-matched sibling
donor hematopoietic cell transplants performed as therapy for 10
CML patients in 1982, and subsequently published a larger study on HLA-identical siblings, the 4-year probabilities of survival and EFS
167 patients transplanted from matched siblings through 1983. were 49% and 43%, respectively, and the probability of relapse was
Long-term follow-up demonstrates that approximately 40% of 12%. The outcome of transplantation for patients in BC is poor, with
patients transplanted in CP nearly 20 years ago are surviving. HCT a high rate of disease recurrence and transplant-related deaths with
became the first curative treatment in CML. Data from 4267 recipi- EFSs of 43%, 18%, and 11% at 100 days, 1 year, and 3 years,
ents of matched sibling transplants reported to the Center for respectively. Before the development of imatinib, only a small propor-
International Blood and Marrow Transplant Research (CIBMTR) tion of patients with blast-phase CML (generally, patients with
between 1994 and 1999 show a probability of survival of 69% ± 2% lymphoid BC) could achieve a hematologic remission with chemo-
for 2876 patients transplanted within the first year from diagnosis, therapy. Response rates of patients with CML in blast phase to
and 57% ± 3% for 1391 patients transplanted more than 1 year from imatinib are considerably higher than those seen with conventional
diagnosis. Contemporary results from selected single institutions chemotherapy. These responses tend to be short, particularly in the
continue to demonstrate excellent outcomes with HCT, in part setting of lymphoid BC. The German CML Study Group reported
because of advances in preparative regimens, supportive care, and 3-year respective OS rates in selected high-risk CP, imatinib-failure
HLA typing for unrelated donors (Fig. 67.8). For example, the Seattle CP, and AP/BP patients after allogeneic HCT of 88%, 94%, and
group, using a preparative regimen of targeted BU plus cyclophos- 59%, supporting its use as a second-line treatment following TKI
phamide (CY) in 131 consecutive CP CML patients, reported survival failure.
at 3 years posttransplant of 86%, with 87% of surviving patients Preparative regimens have improved incrementally. The majority
molecularly negative for BCR-ABL. of patients treated in the early 1980s received a preparative regimen
The outcome of allogeneic HCT in CML is influenced by many of 120 mg/kg CY, followed by total-body irradiation (TBI). Tutschka
factors, including the phase of disease, type of donor used (related or and colleagues later described the use of 16 mg/kg BU administered
unrelated), the source of the stem cell product (marrow or peripheral over 4 days combined with 60 mg/kg CY on each of 2 successive
blood), and the age of the patient. Outcomes are superior for HCT days, in myeloid malignancies. In 1988 a randomized trial of BU/CY
in CP compared with advanced phases of disease. In a Seattle analysis versus CY/12 Gy TBI in myeloid malignancies showed no differences
of 58 patients with AP CML who received transplants from between the CY/TBI and BU/CY treatment groups in survival at 3